Previous Issues Volume 2, Issue 1 - 2017
Diagnosis and Management of Knemidocoptes Pilae in Budgerigars (Melopsittacus Undulates): Case Reports In Egypt
Mohamed E. Abou-Alsoud1, Gamal I. Karrouf2, 3
Faculty of Meteorology, Environment and Arid Land Agriculture, King Abdulaziz University, Jeddah 21589, Saudi Arabia. 2
Faculty of Science, Medical Physics Department, King Abdulaziz University, Jeddah 21589, Saudi Arabia.
3 Surgery, Anesthesiology and Radiology Department, Faculty of Veterinary Medicine, Mansoura University, Mansoura, 35516 Dakahlia, Egypt.
Corresponding Author: Gamal I Karrouf, Faculty of Science, Medical Physics Department, King Abdulaziz University, 21589 Jeddah, Saudi Arabia, and Surgery, Anesthesiology and Radiology Department, Faculty of Veterinary Medicine, Mansoura University, Mansoura, 35516 Dakahlia, Egypt, Tel: 966 6472033; Email: [email protected] 1
Received Date: 01 Dec 2016 Accepted Date: 30 Dec 2016 Published Date: 03 Jan 2017
Copyright © 2017 Karrouf GI
Citation: Abou-Alsoud ME and Karrouf GI. (2017). Diagnosis and Management of Knemidocoptes Pilate in Budgerigars (Melopsittacus Undulates): Case Reports In Egypt. Mathews J Vet Sci. 2(1): 007
ABSTRACT
An outbreak of knemidocoptic mange caused by Knemidocoptes pile (K. pile) is described in caged budgerigars (Melopsittacus undulatus) in Mansoura public park, Egypt. The present study was conducted to detect the etiological parasites (Knemidocoptes pile) by documented clinical findings, skin scraping and histopathologic changes of the affected regions. 19 individuals out of 165 (11.5 %) displayed obvious lesions of progressive dermatitis, various degrees of scaly epithelial proliferation, thick yellow crusts and loss of feather areas. Most of the cases showed a decrease in body weight due to feeding difficulties and anorexia. When the feet and face were infected; lameness was noticed in advanced chronic cases with sometimes blindness and as a result of infection scaly face and leg had occurred. Clinical illness of K. pile infection disappeared 7-20 days after a single subcutaneous injection of 0.02-0.1 ml of ivermectin. Another alternative simple household treatment was applied.
KEYWORDS Knemidocoptes Pilate; Mange; Melopsittacus Undulates; Skin Scraping.
INTRODUCTION
Knemidocoptes are mites burrow into unfeathered skin in birds, causing unsightly, uncomfortable, and potentially life-threatening lesions. The spread of these mites occurs from prolonged close contact between birds, such as occurs between mothers and unfeathered young. Three main species of Knemidocoptes affect birds and these are K. mutants, K. gallinae and K. pile, which all cause different disease manifestations and clinical signs [1-3]. Knemidocoptes pile predominantly parasitizes psittacine birds. Most reports involve budgerigars (Melopsittacusundulatus). Knemidocoptiasis was first described in budgerigars and has also been found in Alexandrine parakeets [4]. It has also been seen in several South American birds, such as green-winged macaws (Arachloroptera spp) and Amazon parrots (Amazona spp.) [5]. Knemidocoptes pile is responsible for scaly face mange in budgerigars and is caused when the mites burrow into feathered parts of the beak and the lightly feathered areas of the face and body leading to loss of feathers and scaliness around the base of the beak and spreading over the face [6, 7]. The aim of this research is to evaluate a diagnostic model for uncovering Knemidocoptes pile in Melopsittacus undulates based on clinical findings, skin scraping and histopathological changes of a KSF versus a CF, in an attempt to adopt the model in future regional surveillances.
MATERIALS & METHODS
Two groups of budgerigars (Melopsittacus undulatus) weighing 35-40 gm were included in this research, located at Mansoura region of subtropical Egypt. The first flock consisted of 165 budgerigars showing symptoms and gross lesions of Knemidocoptes, labeled as ”Knemidocoptiasis suspected flock” (KSF), and the other herd consisted of 100 budgerigars, showing no symptoms either grossly or by skin scraping and histopathological examinations, labeled as ”control Flock” (CF). Records of signs in the two flocks were documented, and signs of the suspected condition were recorded. Deep skin scrapings were taken from the lesions with a small scalpel or a spatula, carefully obtain a small sample of keratinized debris, scales, and crusts from the affected area, and subsequently rinsing with Amman’s lactophenol. And macerated in 10% KOH. Then the sample onto a slide pre-moistened with a drop of mineral oil. The identification of the parasite seen in the scrapings was based on its morphological peculiarities [8].
Histopathological study
Resin embedded ultrathin sections were done by special microtome from infected beak and legs were taken and stained with modified Ziehl-Neelsen [9]. Techniques using kinyoun’s carbolfuchsin and malachite green stain as a counterstain for one minute. It is a new record of a rapid technique for procurement and staining the affected parasites in tissues. Also staining with H and E were used in addition.
RESULTS
Clinical findings
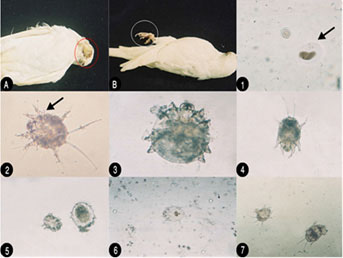
The clinical findings showed an 11.5 % infection in the” Knemidocoptes suspected flock” (KSF), versus its absence in the control flock (CF). In the present study, 19 out of 165 (11.5 %) examined budgerigars (Melopsittacus spp.) were found to be infected with K. pile (11.5%) and developmental stages of the K. pile were showed. Eggs, a six-legged larva, eight-legged nymph appears. Following one or more skin molts the sexually mature adult mite was formed. The Melopsittacus undulatus in the KSF showed symptoms of itching and proliferative, spongy-like lesions of various severities. The lesions were localized on the bare skin around the cere, beak in 10 out of birds accompanied by scaly hyperkeratosis with differing degrees of beak malformations and on feet in 6 and around eyes in 3. According to the information gained from the owners. Most of the diseased birds revealed marked loss of weight and body condition. The infections had appeared in some cases in conjunction with stress such as the movement of birds to another place or a new cage. The microscope examination of the scraping revealed an abundance of mites. All eggs, larvae, and females of K. pile were detected in the crust, but males were not found. K. pile was quite small, mostly covered with soft translucent skin. Their body was globose. Two long bristles are found on the posterior margin of the abdomen. The legs: four pairs of legs for adults and nymphs, three pairs for larvae were provided with Bell shape sucker or suctorial cups on stalks (pedicels) on the tarsi ornamented by hairs (setae). The pedicles of the tarsal suckers were shorter. The female: have no suckers on any of the legs. Legs 3 and 4 of the females only end in bristles instead of suckers (caruncles). The males: has no adrenal suckers (copulatory discs) and its posterior margin of the abdomen is not bi-lobed (Figure 1)
Figure 1: (a) Gross lesions Australian bird showing lesion on face with progressive dermatitis; B. Showinglesion on the feet with obvious lesion of epithelial proliferation; (b) K. pile developmental stages (microscopically); (1) Ova of the parasite K. pile in skin scrape; (2) Larva showed bell-shaped sucker (caruncles) or suctorial cups; (3) None gravid adult female has no suckers on any of the legs; (4) Larva shows shorter pedicels of the tarsal sucker; (5) Nymph in the left and adult female; (6) K. pile nymph; (7) Larvae showed globose body with two long bristles on the posterior margin of the abdomen.
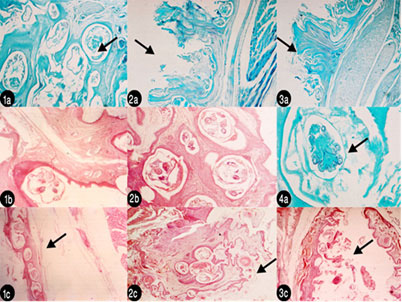
Histopathological findings of KSF showed mild perivascular cell infiltration and edema was seen in the reticular layer of the hyperplastic dermis. No male mites were observed. Lesions have been described when mite invade feather follicles, skin folds and the epidermis by direct penetration, causing pouchlike cavities and also producing secondary pouches, resulting in a lesion “honeycombed” by many cavities. Mites cause papillomatous proliferation of the epidermis (acanthosis) and cystic degeneration of the feather follicles. Both orthokeratotic and parakeratotic hyperkeratosis are seen and contribute to superficial keratin sloughing. Mites are seen within all layers of the stratum corneum in heavy infestations, as demonstrated in the histologic sections then reach the deeper layers of stratum corneum, causing hyperkeratosis, sloughing of superficial keratin (Figure 2).
Figure 2: Histopathological lesions (1a): Deep layer in leg epidermis showing all burrowing mites embedded in dermis and cause lesion like cavities and different stages of K. pile x 500 stained with malachite green 1%; (2a) Ultrathin section in leg showing hyperplastic dermis with mild perivascular cell infiltration and edema in the reticular layer x 125 stained with malachite green 1%; (3a) ultrathin section in beak showing the same pathogenesis like happened in leg x 125 malachite green; (1b) H&E histopathological section in beak x 500 showing pouch-like cavities; (2b) Ultrathin section in beak stained with DMSO- x – 500 showing many gravid females forming “honeycombed” lesion of many cavities; (4a) Ultrathin section stained with malachite green x-500 ventral aspect of larva in deep layer of stratum corneum; (1c) H&E –x- 125 in leg and its muscle.
DISCUSSION
The clinical findings in the two compared flocks of Melopsittacus undulatus based on the presence in the first herd of 11.5 % infestations and symptoms conforming with the Knemidocoptiasis, and the absence of infection and symptoms in the control flock(CF), helped in the presumptive diagnosis of the affected birds as belonging to a” Knemidocoptiasis suspected herd” (KSH). The acceptance of the hypothesis that the designed diagnostic model in this research could lead to a final diagnosis of Knemidocoptiasis, depended on gross examination skin scraping and histopathological examinations of samples collected from the Melopsittacus undulatus of the KSH and CF [ 9,10]. Cases of ”scaly leg” caused by K. pile reported in the present case have been described in India where, as well as an Overgrowth of the beak very similar to that described by us, there were also obvious alterations around the nostrils [11, 12]. Likewise, in Turkey, an outbreak of mite infestation was described in a group of 30 budgerigars, causing pruritus and proliferative spongiform lesions of differing degrees of severity, with lesions mostly on the face and beak, although also on the legs and, to a minor degree, around the eyes [13]. Scaly face in the budgerigar is considered pathognomonic for knemidocoptiasis. A diagnosis is usually easily confirmed by identifying the mites on a skin scraping under the microscope. The mites are recognized by their globoid shape and stubby legs that barely extend beyond the body's lateral margins. The same lines are used in the present study [14]. It is recorded that K. pile infection may remain latent for a considerable time until stress occurs, such as chill or movement to a strange cage [15]. This parasite has been reported in various countries including Turkeys [16-18]. The pathogenic effect arises from the mechanical (burrowing into tissues) and chemical action (metabolites from their excretions) of the mites. As a general rule, knemidocoptic mange is transmitted by direct contact and it mainly affects young individuals with nutritional deficiencies, with high levels of stress or poor conditions of hygiene [13]. Although the disease is also associated with immune-depression and degree of consanguinity [19]. The pathogen may also enter the bird cage via contact with free-living birds that are attracted to the surrounds by the bird feeders or the caged birds themselves. Our observations about occurrence of knemidocoptic mange in some birds, which are under stress, are in line [15]. The microscopic examination of skin scrapings confirmed the presence of K. pilae. Clinical inspection showed that all the birds had itching and proliferative, spongy-like lesions of various severity. The lesions were localized on cere, beak, feet and in eyes [7, 8, 15]. Parasitisation by K. pilae is identified as the origin of the deformations found in the beak. Beak deformities with overgrowth or shortening of the maxilla that preventing their correct alignment are rarely observed phenomena [20, 21]. These anomalies have a varied etiology, amongst which are trauma, abnormal wear of the rhamphotheca [22], nutritional deficiencies (vitamins and calcium metabolism), tumors and those caused by mange mites by the Knemidocoptes species, which are easily transmitted by direct contact between caged birds, and in many cases affect other parts of the body such as the legs [7, 10, 11, 22, 23]. Generally, the reported beak malformations coincide with previous findings in Turkey [13]. However, K. pilae mange in the present study causes proliferative spongiform lesions in areas without feathers, such as the legs, nostrils, face, and around the eyes [7]. The diagnostic model was further strengthened by the results of the histopathologic study, showing the absence of gross or microscopic lesions in the CF and the presence of the specific Knemidocoptiasis lesions in different examinations of the KSF where the Budgerigars of the KSF had for histologic examination will yield more mites. Mites cause papillomatous proliferation of the epidermis and cystic degeneration of the feather follicles. Both orthokeratotic and hyperkeratosis are seen and contribute to superficial keratin sloughing (Figure 1) [24, 25].
CONCLUSION
The obtained data and those reported in literature allows for accepting the hypothesis of implementing this model for reaching to a final diagnosis of Knemidocoptiasis in Melopsittacus undulates . It is recommended in the near future to include this diagnostic model in the national surveillances of Knemidocoptiasis in Melopsittacus undulates of the subtropical areas of the Middle Eastern countries, and other regions of the world that are experiencing similar signs in their Knemidocoptiasis suspected flocks.
REFERENCES
- Taylor MA, Coop RL and Wall RL. (2007). Veterinary Parasitology Blackwell Publishing.
- Saif YM. (2008). Disease of Poultry (Twelfth edition) Blackwell Publishing.
- Jordan F, Pattison M, Alexander D and Faragher T. (1999). Poultry Disease (Fifth edition), W.B. Saunders.
- Keymer IF. (1983). Parasitic diseases. In: Petrak ML, ed. Diseases of cage and aviary birds. 2nd ed. Philadelphia, Pa: Lea & Febiger, 583-587.
- Bauck L. (1997). Avian dermatology. In: Altman RB, Clubb SL, Dorrestein GM, et al, eds. Avian medicine and surgery. Philadelphia, Pa: WB Saunders Co, 549.
- Hochleitner M. (1992). Diagnosis and Treatment of Avian Skin Diseases. Waltham Focus. 42, 23-30.
- Arends JJ. (1997). External parasites and poultry pests. In: Calnek BW, Barnes HJ, Beard CW, et al, eds. Diseases of poultry. 10th ed. Ames: Iowa State University Press. 805-806.
- Fain A and Elsen A. (1967). Les Acariens De La FamilleKnemidokoptidae, Producteurs De La Gale Chez Les Oiseaux (Sarcoptiformes). ActaZoologicaAntverpiensia. 45, 3-145 (in French).
- Clarke S C and McIntyre M. (1996). Modified detergent Ziehl-Neelsen technique for the staining of Cyclospora cayetanensis. J Clin Pathol. 49(6), 511-512.
- Owen HC, Doneley RJT, Schmidt RE, Patterson-Kane JC, et al. (2007). Keratoacanthoma causing beak deformity in a budgerigar (Melopsittacus undulatus). Avian Pathology. 36, 499-502.
- Rao SR, Ghafoor MA and Narsapur VS. (1967). Cnemidocoptes pilae (Lavoipierre and Griffiths 1951) a causative agent of scaly leg in budgerigar (Melopsittacus undulatus) in India together with the description of the larval forms. Indian Veterinary Journal. 44, 206-208.
- ElbalPA, SalidoVC, Sánchez-Murillo JM, Rabernal FC, et al. (2014). Severe beak deformity in Melopsittacusundulatus caused by Knemidocoptes pilae. Turk J Vet Anim Sci. 38(3), 344-346.
- Toparlak M, Tüzer E, Gargili A and Gülanber A. (1999). Therapy of knemidocoptic mange in budgerigars with spot-on application of moxidectin. Turk J Vet AnimSci. 23, 173-174.
- Greve JH. (1986). Parasitic diseases. In: Fowler ME, ed. Zoo and wild animal medicine. 2nd ed. Philadelphia, Pa: WB Saunders Co. 238.
- Urquhart GM, Armour J, Duncan JL, Dunn AM,et al. (1987). Veterinary Parasitology, Longman Sci. and Technical. Essex, UK.
- Schulz TA, Stewart JS and Fowler ME. (1989). Knemidocoptes mutants (Acari: Knemicoptidae) in a Great-horned Owl (Bubovirginianus). J. Wildlife Dis. 25(3), 430-432.
- Mallo O. (1991). Use of Ivermectin as Local Treatment of Knemidecoptic Mange in Canaries. Veterinaria Argentina. 8, 187-189.
- Tüzer E, Toparlak M and Arslan MÖ.(1995). Therapy of Knemidocoptic Mange in Budgerigars With Intramuscular Injection of Ivermectin Tr. J. of Veterinary and Animal Sciences. 19, 131-132.
- Godoy SN. InCubas ZS, Silva JC and Cato-Dias JL. (2006). Tratado de Animais Selvagens. So Paulo: Roca, 16, 222 (in Portuguese).
- Sogge MK and Paxton EH. (2000). A summary of observed physical deformities in the Willow Flycatcher: 1996-2000. Flagstaff, Arizona: Forest and Rangeland Ecosystem Science Center.
- Verea C and Verea JM. (2010). Deformidad del picoen el azulejo de jardnThraupisepiscopus(Passeriformes: Thraupidae) de Venezuela. RevistaBrasileira de Ornitologia. 18, 64-67.
- Olsen GH. ( 2003). Oral biology and beak disorders of birds. The Veterinary Clinics: Exotic Animal Practice. 6, 505- 521.
- Clayton DH, Moyer BR, Bush SE, Jones TG, et al. (2005). Adaptive significance of avian beak morphology for ectoparasite control. Proceedings of the Royal Society of Biology. 272, 811-817.
- Schmidt RE, Reavill DR, and Phalen DN. (2003). Integument. In: Pathology of pet and aviary birds. Ames: Iowa State University Press, 178-179.
- Morishita TY, Johnson G and Johnson G. (2005). Scaly leg mite infestation associated with digit necrosis in bantam chickens (Gallus domesticus). J Avian Med Surg. 19, 230-233.