Previous Issues Volume 2, Issue 1 - 2018
Postoperative Scheduled Intravenous Acetaminophen in Patients Undergoing Laparoscopic Cholecystectomy in Japan
Hideki Taniguchi1,Masaki Takenouchi1,Masaharu Imaura1,Mari Nagahuchi2,Hideki Tachibana2,Takaaki Kamada2,Tomoyuki Sato2,Kan Handa3, Tomohisa Egawa3
1Perioperative Support Center, Saiseikai Yokohama-shi Tobu Hospital
2Department of Anesthesiology, Saiseikai Yokohama-shi Tobu Hospital
3Department of Gastrointestinal Surgery, Saiseikai Yokohama-shi Tobu Hospital
Corresponding Author: JOsamu Nishida, Department of Anesthesiology and Critical Care Medicine, Fujita Health University School of Medicine, 1-98 Dengakugakubo, Kutsukake-Cho, Toyoake-city, Aichi, 470-1192, Japan, Tel: +81-562-93-9008; E-Mail: [email protected]
Received Date: 30 Dec 2017 Accepted Date: 09 Jan 2018 Published Date: 12 Jan 2018
Copyright ©2018 Taniguchi H
Citation: Taniguchi H, Takenouchi M, Imaura M, Nagahuchi M, et al. (2018). Postoperative Scheduled Intravenous Acetaminophen in Patients Undergoing Laparoscopic Cholecystectomy in Japan. M J Anes. 2(1): 009.
ABSTRACT
Background: This study aimed to assess the economic burden and effect of scheduled intravenous acetaminophen (SIVA) on the analgesic activity in patients undergoing laparoscopic cholecystectomy (LC) following our hospital"s management protocol.
Methods: Patients undergoing LC were enrolled prospectively and consecutively, and managed using the old protocol (Group C), and managed following the new protocol using SIVA (Group S). The primary outcome was postoperative pain assessment estimated by the Numerical Rating Scale (NRS) on a postoperative day 1. Secondary outcomes were the number of rescue medicines and the length of hospitalization (LOH).
Results: The NRS was lower in group S than in group C (group S; 1.0 [0,0–2.75,8.0] vs. group C; 4.0 [0,05.75,6.0]: P < .0001). The number of rescue medicines was lower in group S compared to group C (0 [0,0-0,3.0] vs. 1.0 [0,0-2.25,5.0]: P < .0001). LOH after surgery was also shorter in group S than in group C (3.0 [3.0,3.0-3.0,7.0] days vs. 3.0 [3.0,3.0-3.0,12.0] days: P = 0.0462)
Conclusions: : Introduction of SIVA into the postoperative pain management protocol of LC reduced the postoperative NRS, the number of rescue medicines and the LOH. We recommend the standardization of postoperative pain management in clinical management protocols in Japan.
KEYWORDS
Scheduled Intravenous Acetaminophen; Acetaminophen; Postoperative Pain Laparoscopic Cholecystectomy; Clinical Management Protocols Enhanced Recovery After Surgery (ERAS).
INTRODUCTION
Evidence-based guidelines for the perioperative care of colon [1] and rectal [2] surgery were published by the enhanced recovery after surgery (ERAS) society.
The guidelines recommend minimizing opioids for an earlier return of bowel function and a shorter length of hospitalization (LOH) [3]. One of the simplest methods to limit opioid intake is to schedule narcotic alternatives, such as acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and gabapentin, rather than giving them on an as-needed basis [4]. The scheduled use of non-selective or selective NSAIDs, cyclooxygenase (COX) 2 inhibitors, and acetaminophen (orally or intravenously [4]) have been shown to improve postoperative analgesia and reduce the consumption of systemic opioids as well as their dose-dependent adverse effects [5,6]. A meta-analysis of 30 randomized controlled trials (RCTs), including 2364 patients, showed that the use of IV acetaminophen given either before surgery or before the arrival to the post-anesthesia care unit reduced the risk of nausea and pain, which were shown to delay surgical recovery [6].
In 2011, the clinical doses of IV acetaminophen in Japan increased from a maximum of 1500 mg to 4000 mg per day to allow for an optimal analgesic effect. Moreover, it was only prescribed as needed based on the pain levels with no special recommendations for the scheduled prescription to postoperative patients. This study aimed to assess the economic burden and effect of scheduled intravenous acetaminophen (SIVA) on the analgesic activity in patients undergoing laparoscopic cholecystectomy (LC) following our hospital's management protocol. Based on the results, SIVA can be introduced into the management protocols of several procedures in Japan. We chose LC as it would allow us to assess the isolated effect of SIVA on pain control without the effect of epidural anesthesia.
MATERIALS AND METHODS
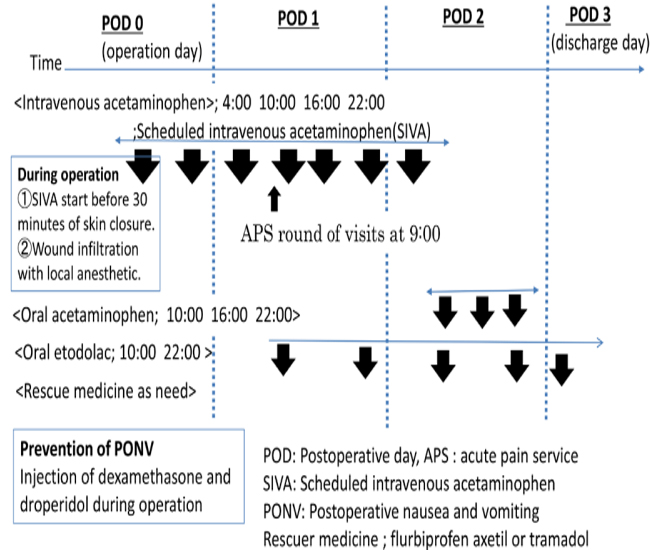
The present study was approved by the Institutional Review Board of Saiseikai Yokohama-shi Tobu Hospital (Study-2017092), registered with the Center for Clinical Trials, Japan Medical Association (JMA) Clinical Trials Registry (JMA registration number: JMA-IIA00317), and conducted after disclosure of information. Subjects included were patients scheduled to undergo elective LC for gallstone, inflammation of the gall bladder (GB), or GB polyp. In our hospital's LC management protocol, patients are discharged on the morning of postoperative day 3. Also, NSAIDs, tramadol, or acetaminophen are conventionally used randomly and as needed (table1). On February 1, 2017, our hospital included SIVA in a new LC postoperative management protocol (Table1, Figure1).
Table 1:: Management protocol of laparoscopic cholecystectomy
| Period | The old protocol (Group C) | The new protocol (Group S) |
|---|---|---|
| Preoperative | Major component Intake of oral rehydration solutions until 2 hours before surgery No laxative medication and premedication Thorough oral cavity care | |
| Intraoperative | Use of short-acting anesthetics Keeping the patient warms Intravenous acetaminophen was injected 30 min before the end of surgery. Wound infiltration with local anesthetic is performed. Prevent of PONV during operation | |
| Postoperative | Early ambulation and oral diet (POD 1) On an operative day, intravenous infusion of acetaminophen, flurbiprofen axetil or tramadol are taken as needed repeatedly. | Early ambulation and oral diet (POD 1) Scheduled intravenous acetaminophen and as-needed basis administration of NSAIDs. |
Information sharing/patient education (before and after surgery)
b500-mL plastic bottles (OS-1, classified as food in Japan. Otuka Pharmaceutical Factory, Inc., Tokushima, Japan). We provided 1500 ml of the solution to the patients at 20:00 on the night before surgery and allowed them to drink it at least 500 mL at any time they wanted until 2 hours before entering the operating room
c While the body surface was covered with blankets or towels, the patients were warmed with Bair Hugger (3M, St. Paul, MN) set at 38 degrees C. The target body temperature was set at a bladder temperature of 36 - 37 degrees C. The fluids used were warmed to 35 -36 degrees C with a warming device before transfusion
Figure 1: A new laparoscopic cholecystectomy postoperative management protocol.
IV acetaminophen was injected 30 min before the end of surgery and every 6 h for 48 h, with a total of 7 doses, at doses of 1000 mg for patients weighing = 50 kg, and 15 mg/kg for patients weighing < 50 kg. Wounds were infiltrated with a local anesthetic (0.2% ropivacaine; 20–40 ml).On a postoperative day, 1 patient was given oral etodolac, on postoperative day 2 acetaminophen was changed from IV to oral from.
The switchover to the new protocol took place in a transition period between February 1 to 28, 2017. Between August 1, 2016, and January 31, 2017, patients undergoing LC were enrolled prospectively and consecutively and managed using the old protocol. They were designated as the control group (Group C). Those enrolled between March 1, 2017, and August 31, 2017, were managed following the new protocol using SIVA in the initial 24-h period, and they were designated as the study group (Group S).
The switchover to the new protocol took place in a transition period between February 1 to 28, 2017. Between August 1, 2016, and January 31, 2017, patients undergoing LC were enrolled prospectively and consecutively and managed using the old protocol. They were designated as the control group (Group C). Those enrolled between March 1, 2017, and August 31, 2017, were managed following the new protocol using SIVA in the initial 24-h period, and they were designated as the study group (Group S).
The inclusion criteria included patients aged 20 years or older classified as class I or II by the American Society of Anesthesiologists Physical Status (ASA-PS) scale. The exclusion criteria included patients with renal dysfunction (estimated creatinine clearance < 20 ml/min or ongoing hemodialysis), liver dysfunction (AST or ALT > 100 U/L), and/or deviating from our hospital's LC management protocols.
This was a retrospective observational study comparing patients following two different management protocols (with and without SIVA) (table1). The primary outcome was pain score on postoperative day 1. The secondary outcomes included the LOH and the incidence of postoperative nausea and vomiting (PONV) from postoperative day 0 to 1.
Primary outcome Postoperative pain assessment was estimated by the Numerical Rating Scale (NRS) on postoperative day 1 at 9:00 AM at rest. A pharmacist estimated the NRS from patients during Acute Pain Services (APS) rounds.
Secondary outcomes The postoperative use of non-opioid analgesics was assessed by calculating the frequency of prescribed analgesics including NSAIDs, tramadol, and acetaminophen. The incidence of PONV was assessed by calculating the frequency of prescriptions of metoclopramide, droperidol, and dimenhydrinate. Data on sex, age, body mass index (BMI), ASA-PS, disease type, maintenance of anesthesia, operation time, and LOH) were retrieved from the database.
Operative procedure Four ports were used including a 10 mm optical, two 5 mm operating, and a 5 mm assisting ports. The optical port was placed at the umbilicus and a flexible laparoscope was routinely used. The fundus of the GB was grasped by the assistant and flipped upwards and over the superior edge of the right lobe of the liver. The triangle of Calot and the cystic duct’s infundibular junction were identified. The infundibulum was retracted info-laterally, whereas the fundus was retracted towards the right shoulder. The plane of dissection of Calot’s triangle was anterior and above to Rouvier’s Sulcus. The medial areolar tissue between the liver and infundibulum was divided. The inferior part of the GB was freed from the GB fossa. The cystic duct and artery were defined, and Strasberg’s Critical view of Safety was established. The cystic duct and artery were clipped then divided by scissors. The superior part of the GB was separated from the liver and extracted through the 10-mm umbilical port.
Maintenance of anesthesia No pre-anesthetic medications were administered. Anesthesia was induced with propofol (1-2 mg/kg) and remifentanil (0.5 μg/kg/min). Following muscle relaxation with rocuronium (1 mg/kg), the trachea was intubated. Anesthesia was maintained with inhalation anesthesia (4-5% desflurane or 1-1.5 % sevoflurane) without nitrous oxide and remifentanil, while fentanyl and rocuronium were intermittently administered as needed. IV acetaminophen was injected 30 min before the end of surgery and every 6 h, at doses of 1000 mg for patients weighing = 50 kg, and 15 mg/kg for patients weighing < 50 kg. Wounds were infiltrated with a local anesthetic (0.2% ropivacaine; 20-40 ml). After completion of the surgical procedure, the remifentanil and inhalation anesthesia was stopped, and muscle relaxation was reversed. When the swallow reflex recovered, the patient could follow our directions and grasp our hand, the spontaneous breathing rate was = 12 breaths/min, end-tidal CO2 was < 45 mmHg, and SpO2 was = 95%, the endotracheal tube was removed. Epidural and total IV anesthesia (continuous infusion of propofol and remifentanil) was not selected for this surgery.
Postoperative pain management In a group, C medicines were taken as needed. When the patient's pain was over 4 at rest on NRS, the nurse would administer one rescue medication from the ordered medications by the physician. The first-choice drug was acetaminophen, the second was an NSAIDs, and the third was tramadol. On the day of the procedure, IV infusion of acetaminophen (Acelio IV Injection; Terumo Corporation, Tokyo, Japan), flurbiprofen axetil (Ropion IV Injection; Kaken Pharmaceutical Co., Tokyo, Japan) or tramadol (Tramal IV Injection; Nippon Shinyaku Co., Kyoto, Japan) was performed as needed. On a postoperative day 1, oral acetaminophen, NSAIDs, or tramadol were provided as needed. The medicines were administered at intervals of over 4 h.
In group S, patients were given all medications in a standardized manner receiving a SIVA every 6 h, for 48 h, with a total of 7 doses (1000 mg/dose every 6 h for patients weighing with patients weighing = 50 kg, and 15 mg/kg/dose every 6 h for patients weighing < 50 kg). On a postoperative day, 1 patient was given oral etodolac, on postoperative day 2 acetaminophen was changed from IV to oral from. When a patient's pain was over 4 at rest on the NRS, the nurse would administer one rescue medication from the ordered medications by the physician. The first-choice drug was an NSAIDs, and if kidney dysfunction or symptoms originating from a digestive organ were observed, then tramadol was chosen. The medicines were administered at intervals of over 4 h. We drew a sample to test for AST and ALT on the morning of postoperative day 1. If the values of AST and ALT were greater than 100 U/L, then the SIVA was stopped.
Prevention of PONV In both groups, patients with high PONV risk (? 60%; scoring system by Apfel et al. [7]) were given antiemetics during the procedure. Dexamethasone (6.6 mg) was injected after induction of anesthesia and droperidol (0.625–1.25 mg) was given at the end of surgery. When PONV occurred, rescue therapy with antiemetics was provided (metoclopramide, droperidol, or dimenhydrinate).
Statistical analysis Statistical analysis was performed with JMP10.0.2 (SAS Institute, Cary, NA). For categorical variables, the frequency (number and background of patients) was determined for each group. The results of the two groups were compared by Fisher’s exact test for binary categorical variables. NRS, LOH and the number of medicinal use were compared between the groups and expressed with a median value (minimum value, bottom quartile, top quartile, maximum value), for which Wilcoxon rank-sum test was used. For continuous variables, results were expressed with a mean value, standard deviation (SD), for which a two-sample t-test was performed. The detailed significance level (P-value) was set at 5%.
Discharge criteria The discharge was on the morning of postoperative day 3 based on the management protocol. Patients were discharged when they met the following criteria: (a) had good pain management with oral analgesia; (b) were able to have solid food without abdominal symptoms; (c) were independently mobile or had the same mobility level as prior to their admission; (d) met all of the above and were willing to go home.
DATA PREPARATION
The synchronization between both devices was ensured by annotating the exact start time given by the two devices. Data values of cardiac output were not taken into consideration when there was an instantaneous variation of either HR, CO or SV greater than 25% which was not justified from a clinical point of view. This led to a total rejection rate of < 2% for LiDCOrapid and < 1% for CCO. Two patients (one for gastrostomy and another one for cytoreductive surgery) were excluded due to excessive noise, and thus a total of thirteen patients remained to be considered for this study. To create the pairs of cardiac output values, each CO point from CCO was paired with the closest CO point by LiDCOrapid within a time difference of one second between both points.
RESULTS
All patients were treated following a management protocol during the investigation period and were registered. In both groups, eligible patients who met the inclusion and exclusion criteria were selected from medical records of those undergoing elective LC during the study period. As a result, a total of 97 patients, a total of 98 patients, including 54 in group C and 44 in group S, were included in the analysis. Table 2 summarizes the patient characteristics of C- and S-groups.
Table 2: Patient characteristics
| Group C (n=54) | Group S (n=44) | P | |
|---|---|---|---|
| Age (year) | 59.3±116.1 | 60.2±114.4 | 0.766 |
| Sex | 0.2606 | ||
| Male(n) | 27 | 17 | |
| Female(n) | 27 | 27 | |
| Body mass index(kg/ m2) | 24.6±14.6 | 24.5±13.8 | 0.8784 |
| ASA-PS score | 0.6391 | ||
| I(n) | 22 | 20 | |
| II(n) | 32 | 24 | |
| The kind of disease | 0.6169 | ||
| Gallstone(n) | 17 | 16 | |
| Gallbladder polyp(n) | 1 | 2 | |
| Inflammation(n) | 36 | 26 | |
| Maintenance of anesthesia | 0.1134 | ||
| total IV anesthesia (n) | 0 | 2 | |
| Inhalation anesthesia (n) | 54 | 42 | |
| Operative duration(min) | 115.2±140.5 | 113.9±131.3 | 0.861 |
Wilcoxon rank-sum test: significant when p <0.05, ††: Fisher exact probability test: significant when p <0.05, Continuous variables: mean ± standard deviation
Group C: control group, Group S: SIVA group
There were no statistically significant differences in sex, age, ASA-PS, BMI, disease type, or operation time between both groups. With regard to the maintenance of anesthesia, no patient received an epidural; total IV anesthesia was used in two patients. Because there were no exclusion criteria based on the method of anesthesia, these 2 patients were also included. There were no statistically significant differences in the maintenance of anesthesia.
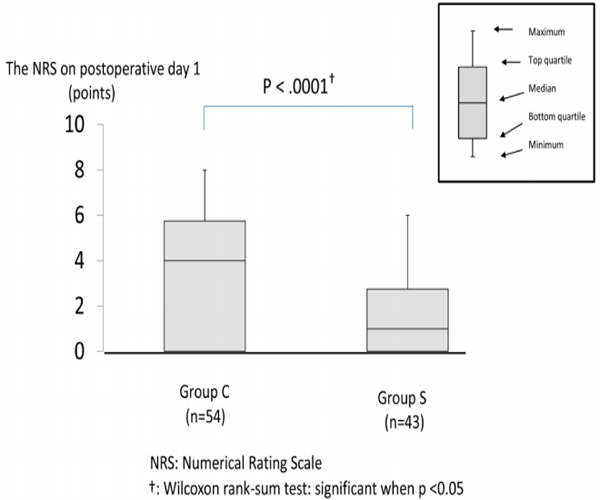
Primary outcome Figure 2 compares the NRS on postoperative day 1 at 9:00 AM between both groups. The NRS was lower in group S than in group C (group S; 1.0 [0,0–2.75,8.0] vs. group C; 4.0 [0,05.75,6.0]: P < .0001).
Figure 2: The NRS on postoperative day 1 at 9:00
The NRS was lower in group S than in group C (group S; 1.0 [0,0–2.75,8.0] vs. group C; 4.0 [0,05.75,6.0]: P < .0001). The NRS was compared between the groups and expressed with a median value (minimum value, bottom quartile, top quartile, maximum value), for which the Wilcoxon rank-sum test was used.
Secondary outcomes The number of rescue medicines was lower in group S compared to group C (group S; 0 [0,0-0,3.0] vs. group C; 1.0 [0,0- 2.25,5.0]: P < .0001) (Figure3).
LOH after surgery was also shorter in group S than in group C (group S; 3.0 [3.0,3.0-3.0,7.0] days vs. group C; 3.0 [3.0,3.0- 3.0,12.0] days: P=0.0462) (group S; 3.1 ± 0.86 days vs. group C; 3.6 ± 1.8 days). In contrast, the incidence of PONV did not differ between both groups (group S; 25.0% vs. group C; 42.6%: P=0.0688). No patients in either group had elevated the levels of AST or ALT of more than 100 U/L on postoperative day 1.
Figure 3:The number of using rescue medicine during postoperative 24hr The number of rescue medicines was lower in group S compared to group C (group S; 0 [0,0-0,3.0] vs. group C; 1.0 [0,0-2.25,5.0]: P < .0001). The number of medicinal use was compared between the groups and expressed with a median value (minimum value, bottom quartile, top quartile, maximum value), for which the Wilcoxon rank-sum test was used.
Table 2: Patient characteristics
| Group C (n=54) | Group S (n=44) | P | |
|---|---|---|---|
| PONV(+) (%) | 42.6% | 25.0% | 0.0688 |
| PONV(-) (%) | 57.4% | 75.0% | |
| LOH after surgery(day) | 3 .0[3.0,3.0- 3.0,12.0] * | 3.0 [3.0,3.0- 3.0,7.0]* | 0.0462† |
†: Wilcoxon rank-sum test: significant when p <0.05
* median value [minimum value, bottom quartile- top quartile, maximum value]
PONV: postoperative nausea and vomiting
LOH: length of hospitalization
DISCUSSION
In this study, postoperative SIVA in patients undergoing LC reduced the NRS on postoperative day 1 at 9:00 AM. Furthermore, it reduced the LOH. During the first 24 h after surgery, postoperative SIVA reduced the number of rescue medicines administered. However, it did not reduce the incidence of PONV
Acetaminophen has a long-standing history and a relatively safe adverse event profile when used at appropriate doses [8]. In November 2010, the US Food and Drug Administration approved the use of IV acetaminophen for the treatment of acute pain and fever in adults and children older than 2 years of age [8,9]. In Japan, we have been able to use IV acetaminophen as well since November 2013 with an increase in the maximum prescribing dose from 1500 mg a day to 4000 mg. However, generally, its use has not been scheduled, with acetaminophen being administered as a one-shot medicine when needed. Therefore, a sufficient and sustained analgesic effect was not usually achieved following surgeries in Japan, leading to the patient's difficulty to get out of bed because of poor pain control. As a result, LOH was longer
ERAS protocol reduces surgical invasiveness, postoperative complications and shortens hospital stay. However, major limiting factors for the ERAS protocol include pain, gastrointestinal (GI) dysfunction, and immobility. Although those 3 factors are interlinked, it is particularly important to control postoperative pain for the improvement of GI dysfunction and immobility. Epidural analgesia has been recommended as a postoperative pain management strategy in the ERAS protocol for major colorectal surgery [10,11]. Though the level of evidence for epidural analgesia during open surgery is high, it has been reported that epidural analgesia is not essential during laparoscopic surgery [1]. However, specifically in laparoscopic surgery, trocar sites are infiltrated with local anesthesia prior to skin closure [12]. The EARS protocol recommends multimodal analgesia (MMA) including opioid, non-opioid, epidural, and local anesthesia for pain management after GI surgery [13].
MMA regimen is based on the routine use of non-opioid analgesia (NSAIDs, COX-2, and acetaminophen) and is indicated in patients undergoing open and laparoscopic abdominal procedures with the aim of reducing the consumption of opioid and their dose-dependent side effects, which delays recovery. For pain management after LC, ERAS guidelines recommend nonopioid and opioid use with dexamethasone and ondansetron, for the prevention of PONV, and not epidural anesthesia [14]. However, in Japan, ondansetron is not used perioperatively because of the lack of insurance coverage. Therefore, in our study, the pain management regimen for group S includes wound infiltration with a local anesthetic and non-opioid analgesics with dexamethasone (6.6 mg) and droperidol (0.625– 1.25 mg) (figure 1).
In this study, we estimated the NRS on postoperative day 1 at 9:00 AM as we wanted to assess the effect of acetaminophen at minimum blood and cerebrospinal fluid (CSF) concentrations. Previous research has shown that the concentrations in the blood and CSF are minimum at this time. Had acetaminophen been administered immediately, the pain would have been uniformly suppressed. We believe that the reason that the NRS and the number of rescue medicines administered during the first 24 h after surgery were lower in group S compared to group C was that the CSF concentration of acetaminophen was maintained by the SIVA. Neil K et al demonstrated that earlier and greater CSF penetration occurs as a result of the earlier and higher plasma peak levels achieved with IV acetaminophen administration compared with those administered orally or rectally [15]. Studies showed that IV acetaminophen reaches a higher peak plasma concentration compared to when administered orally. In addition, IV administration does so faster [16]. SIVA has repeatedly been shown to be safe and efficacious analgesia in major orthopedic surgeries [17,18]. Similarly, it was efficacious in gastrointestinal and gynecological surgeries [19,20]. Although the incidence of PONV was not different between both groups in our study, we believe the reason to be the limited use of antiemetics perioperatively in Japan. Had we used ondansetron in this study, the incidence of PONV might have decreased as in other studies on LC [21,22].
Our results showed that the LOH following surgery shortens by merely introducing SIVA in LC managed ERAS protocols. In Japan, the LOH following surgery can be reimbursed for a relatively long period of time, which can affect LOH after surgery. Because of this, the primary endpoint surgical studies in Japan are not LOH following surgery. However, in our study, we believe that SIVA reduced postoperative pain and patients whose discharge was postponed was due to the lack of pain control. Santoso JT et al. demonstrated that multimodal pain control was associated with a significant reduction in the LOH following open abdominal hysterectomy [23]. Similarly, Shaffer EE et al. demonstrated that opioid use including SIVA for postoperative pain management had the potential to decrease LOH, opioid-related complication rates, and hospital expenses [24]. Because this study did not include the use of opioids in either group, we concluded that the effect of analgesia induced by SIVA reduced the LOH. In Japan, where the LOH following surgery is difficult to use as a primary endpoint, its shortening is remarkable. We recommend the standardization of postoperative pain management in the clinical protocols in Japan. In the future, we expect the evaluation of SIVA in other types of procedures.
This study had several limitations. First, it was a retrospective observational study and not an RCT. Although the LC surgical procedure was the same in both groups and the perioperative management was standardized, postoperative analgesic regimens differed. As a result, the effect of postoperative SIVA on pain control was noted. Hence, we believe this study to be as informative as an RCT. Second, there was one evaluation time-point for NRS. Ideally, an average of scores at several time-points would have been better. Furthermore, the evaluation of NRS was limited to rest. It would have been preferred to be evaluated while the patients were mobile as well. However, since NRS was evaluated by the same member of APS, its evaluation accuracy was considered high.
Third, in Japan, the LOH is not an index for the medical economic burden. Therefore, the actual economic burden faced by these patients could not be evaluated. DPC/PDPS (Diagnosis Procedure Combination/Per-Diem Payment System) is a Japanese payment system. The medical cost incurred by a patient is the sum of the comprehensive evaluation and the high evaluation set for each DPC. Our results show that the overall cost related to the comprehensive evaluation decreased; however, we could not determine how the high evaluation affected the cost incurred.
CONCLUSION
The introduction of SIVA into the postoperative pain management protocol of LC reduced the NRS on postoperative day 1, the number of rescue medicines administered during the first 24 h following surgery, and the LOH. We recommend the standardization of postoperative pain management in clinical management protocols in Japan.
CONFLICTS OF INTEREST
The authors report no conflicts of interest in this work.
ACKNOWLEDGMENTS
All authors contributed equally to the study design, data collection, data interpretation, figure creation, and writing of the manuscript We would like to thank Editage (www.editage.jp) for English language editing
REFERENCES
- Gustafsson UO, Scott MJ, Schwenk W, Demartines N, et al. (2013). Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 37(2): 259-284.
- Nygren J, Thacker J, Carli F, Fearon KC, et al. (2012). Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/ pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 31(6): 801-816.
- Thiele RH, Rea KM, Turrentine FE, Friel CM, et al. (2015). Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 220(4): 430-443.
- Khoo CK, Vickery CJ, Forsyth N, Vinall NS, et al. (2007). A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg. 245(6): 867-872.
- Amir Aryaie, C. Juergens, C. Ogg, W. Sergent, et al. (2015). Decreased narcotic consumption with the addition of IVacetaminophen in colorectal patients: a prospective, randomized, double-blinded, placebo-controlled study. Dis Colon Rectum. 58 e123.
- Apfel CC, Turan A, Souza K, Pergolizzi J, et al. (2013). Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta-analysis. Pain. 154(5): 677-689
- Apfel CC, Korttila K, Abdalla M, Kerger H, et al. (2004). A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 350(24): 2441-2451
- Pasero C and Stannard D. (2012). The Role of Intravenous Acetaminophen in Acute Pain Management: A Case-Illustrated Review. Pain Manag Nurs. 13(2): 107-124.
- Pickens LA and Meinke SM. (2011). OFIRMEV: a recently introduced drug. J Pediatr Nurs. 26(5): 494-497.
- Fearon KC, Ljungqvist O, Von MM, Revhaug A, et al. (2005). Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 24(3): 466-477.
- Kehlet H and Wilmore DW. (2008). Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 248(2): 189-198.
- Bisgaard T, Klarskov B, Kristiansen VB, Callesen T, et al. (1999). Multi-regional local anesthetic infiltration during laparoscopic cholecystectomy in patients receiving prophylactic multi-modal analgesia: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 89(4): 1017-1024
- Feldheiser A, Aziz O, Baldini G, Cox BP, et al. (2016).Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anesthesia practice. Acta Anaesthesiol Scand. 60(3): 289-334.
- Jensen K, Kehlet H, and Lund CM. (2007). Post-operative recovery profile after laparoscopic cholecystectomy: a prospective, observational study of a multimodal anesthetic regime. Acta Anaesthesiol Scand. 51(4): 464-471.
- Singla NK, Parulan C, Samson R, Hutchinson J, et al. (2012). Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 12(7): 523-532
- . Van der Westhuizen J, Kuo PY, Reed PW and Holder K. (2011). Randomized controlled trial comparing oral and intravenous paracetamol (acetaminophen) plasma levels when given as preoperative analgesia. Anaesth Intensive Care. 39(2): 242-246.
- Sinatra RS, Jahr JS, Reynolds L, Groudine SB, et al. (2012). Intravenous acetaminophen for pain after major orthopedic surgery: an expanded analysis. Pain Pract. 12(5): 357-365.
- Lachiewicz PF. (2013). The role of intravenous acetaminophen in multimodal pain protocols for perioperative orthopedic patients. Orthopedics. 36(2 suppl): 15-19.
- Saurabh S, Smith JK, Pedersen M, Jose P, et al. (2015). Scheduled intravenous acetaminophen reduces postoperative narcotic analgesic demand and requirement after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 11(2): 424-430.
- Herring BO, Ader S, Maldonado A, Hawkins C, et al. (2014). Impact of intravenous acetaminophen on reducing opioid use after hysterectomy. Pharmacotherapy. 34 Suppl 1: 27S-33S.
- Ahsan K, Abbas N, Naqvi SM, Murtaza G, et al. (2014). Comparison of efficacy of ondansetron and dexamethasone combination and ondansetron alone in preventing postoperative nausea and vomiting after laparoscopic cholecystectomy. J Pak Med Assoc. 64(3): 242-246.
- Isazadehfar K, Entezariasl M, Shahbazzadegan B, Nourani Z, et al. (2017). The Comparative Study of Ondansetron and Metoclopramide Effects in reducing Nausea and Vomiting After Laparoscopic Cholecystectomy. Acta Med Iran. 55(4): 254-258.
- Santoso JT, Ulm MA, Jennings PW, and Wan JY. (2014). Multimodal pain control is associated with reduced hospital stay following open abdominal hysterectomy. Eur J Obstet Gynecol Reprod Biol. 183: 48-51.
- Shaffer EE, Pham A, Woldman RL, Spiegelman A, et al. (2017). Estimating the Effect of Intravenous Acetaminophen for Postoperative Pain Management on Length of Stay and Inpatient Hospital Costs. Adv Ther. 33(12): 2211- 2228.