Previous Issues Volume 4, Issue 2 - 2019
The Impact of Normalization Isodose Line (NIDL) on Dose Distribution in Intracranial Tumors Treated with CyberKnife
Zhitao Dai1, Cui Yang2,3, Wei Jiang1, Man Zhao1, Weigang Hu2,3, and Hua Ren1*
1National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China
2School of Physics and Technology, Wuhan University, China
3Department of Radiation Oncology, Fudan University, Shanghai Cancer Center, China
*Corresponding Author: Hua Ren, Department of Radiation Oncology, Cancer Hospital Chinese Academy of Medical Sciences, Shenzhen Center, China, E-mail: [email protected]
Copyright © 2019 Ren H, et al.
Citation: Ren H. (2019). The Impact of Normalization Isodose Line (NIDL) on Dose Distribution in Intracranial Tumors Treated with CyberKnife. Mathews J Cancer Sci. 4(2): 23
ABSTRACT
Objective: Dosimetric characteristics and dose falloff variation of Cyberknife (CK) plans under different isodose lines (IDL) of head tumors were assessed in an attempt to provide reference for clinical radiotherapy treatment planning.
Methods: Ten head tumor patients planned with stereotactic body radiotherapy (SBRT) were selected and replanned, uniformly meeting the same objective with 30Gy covering 95% of the planning target volume (PTV). Four separate plans normalized to 65%, 70%, 75%, 80% IDL respectively were generated for each case. Dose distribution outcomes were compared using dosimetric parameters, including dose-volume histogram (DVH), conformal index (CI), new conformal index (nCI), homogeneity index (HI) and dose gradient index (GI).
Results: Plans normalized to a higher percentage provide a better dose conformation of the prescription dose envelop and PTV homogeneity. As the normalized percentage changes from 80% to 65%, CI changes from 1.16 to 1.24, nCI changes from 1.18 to 1.28, HI changes from 0.25 to 0.54. In addition, GI is defined as the difference between the effective radius of the package volumes of 50% and 100% IDL. For the plans normalized to 65%, 70%, 75%, and 80% IDL, GI values are 6.89 ± 2.60, 7.17 ± 2.72, 7.33 ± 2.84, and 7.57 ± 2.95 respectively. Moreover, it increases as the tumor volume increases.
Conclusion: For SBRT treatment planning, a lower normalized percentage can achieve a better protection of normal tissue, while a higher normalized percentage will result in a greater conformity and homogeneity of dose distribution. Moreover, for different volumes of head tumor, comprehensive consideration should be given to the radiotherapy treatment planning, in order to improve the therapeutic ratio.
Keywords: Cyberknife treatment plan; Normalized isodose line; Dose gradient.
INTRODUCTION
Stereotactic body radiotherapy (SBRT) is a newly-developing tumor radiotherapy method which applies stereotactic technique to radiation therapy [1,2]. Characterized by high dose and few fractions, it achieves the effect of local radical cure of tumors [3]. In theory, the faster delivery modality on the one hand can do devastating damage to the tumors’ DNA in one fraction, on the other hand, it reduces the intrafraction motion during radiation to the max [4,5]. In addition, SBRT technology offers the potential to more closely conform dose distributions to the target so that improves the tumor control probability (TCP) greatly in clinical radiotherapy [6,7], which attracts the attention of many clinical researchers.
Conceivably, more accurate patient positioning and target tracking are requirement because of the single high dose output during SBRT process [5]. Technological advances provide opportunities for precision radiotherapy. Among them, CK is an accelerator that specifically designed for SBRT, which is also known as the stereotactic cyberKnife platform [4]. It is equipped with a six-dimensional robotic arm that enables it to move accurately over a wide range. Moreover, two ceiling-mounted X-ray imaging equipment are equipped as well for position monitoring and tumor tracking [8,9]. These two sensitive devices form a system that achieves the precise dose delivery [10,11]. At present, CK has solved most of the technical problems and enable to perform non-invasive high-precision SBRT on lung, liver, pancreas and brain tumors, bringing new hope to patients [3].
With increasing clinical application of SBRT, the dose distribution of different SBRT plans has become the focus of attention of physicists [8,12]. In addition, it is often difficult to assess the pros and cons of different dose distributions between targets of different sizes and shapes [13]. Therefore, 10 head tumors with various volume and treated by CK were selected in this article. Under the premise of ensuring uniform prescription dose and target area coverage, plans normalized to different IDL were made to study the impact of different IDL and target volume on dose distribution, hoping to provide reference for the design of clinical head tumor SBRT planning.
MATERIALS AND METHODS
Patient selection
10 patients with metastatic head tumor treated by CK in Shanghai Cyberknife research center were chosen randomly for this study. Using GE Discovery CT750HD16 CT scanner to obtain the CT images, with a thickness of 1.5 mm. PTV contouring and organs at risk (OAR) was completed by physicians with 5 years’ experience. The PTV volume is range from 6 cc to 90 cc, while the OAR20 was defined as the site of the tumor extension 20 mm.
Generate treatment plans
For each patients, four separate plans normalized to 65%, 70%, 75%, 80% IDL were generated using MultiPlan4.0.2 planning system. The prescription dose was uniformed to 6Gy*5 with the coverage rate no less than 95%.
Treatment plan evaluation
Dose distributions: The statistical function of the treatment planning software was used to obtain the dose-volume histogram (DVH) of tumor and OAR20. In addition, the mean dose of tumor ( ) and OAR20 (
) and OAR20 ( ) of different plans were also calculated to qualitatively analyze the dose distribution in target and surrounding normal tissues.
) of different plans were also calculated to qualitatively analyze the dose distribution in target and surrounding normal tissues.
Dosimetric parameters: Conformal index (CI), new conformal index (nCI), homogeneity index (HI) and dose gradient index (GI) were calculated to evaluate the quality of the treatment plans under different IDL. Among them, CI and nCI are defined as follows [3,9]:
 is the total volume enclosed by the prescription dose contours,
is the total volume enclosed by the prescription dose contours,  is the volume of PTV area enclosed by the prescription dose contours,
is the volume of PTV area enclosed by the prescription dose contours,  is the volume of PTV. The ideal value for CI (or nCI) is 1. In other word, the closer the CI (or nCI) get to 1, the greater the dose conform to PTV.
is the volume of PTV. The ideal value for CI (or nCI) is 1. In other word, the closer the CI (or nCI) get to 1, the greater the dose conform to PTV.
Moreover, HI is calculated using following formula [11]:
 and
and  represent the doses encompassed 2% and 98% of the PTV respectively.
represent the doses encompassed 2% and 98% of the PTV respectively.  corresponds to prescription dose. Therefore, lower HI means better homogeneity of dose within PTV.
corresponds to prescription dose. Therefore, lower HI means better homogeneity of dose within PTV.
GI is defined as the difference between the effective radius of 50% and 100% isodose package volume, which definition equation is as follows:
 and
and  represent the volume of 50% and 100% IDL packaged respectively,
represent the volume of 50% and 100% IDL packaged respectively,  and
and  represent the equivalent spherical radius of
represent the equivalent spherical radius of  and
and  respectively. It is a dosimetric parameter that has been developed in recent years to evaluate the dose falloff characteristic of different treatment planning [12,14]. As can be seen, lower GI value means faster dose falloff.
respectively. It is a dosimetric parameter that has been developed in recent years to evaluate the dose falloff characteristic of different treatment planning [12,14]. As can be seen, lower GI value means faster dose falloff.
Statistical analysis
The SPSS statistical software was utilized for the statistical analysis. Each parameter was repeated three times and the parameter comparison was carried out using a matching t-test analysis. P<0.05 were considered as statistically significant.
RESULTS
Dose distributions under different IDL
Figure 1 shows the two-dimensional dose distributions in transverse, sagittal and coronal planes of plans normalized to 65%, 70%, 75%, 80% IDL respectively, all exhibit acceptable PTV coverage. The coverage rates are 97.5% ± 0.56%, 97.32% ± 0.64%, 97.31% ± 0.62%, 97.38% ± 0.59% respectively, performing no statistically significance.
Moreover, Figure 2 shows the DVH of PTV and OAR20 towards different IDL. As it illustrates, under different IDL, the prescription dose contours cover roughly the same percentage of PTV. Moreover, the high dose areas appear as the normalized IDL decrease. With regard to OAR20, the dose distribution displays little difference except the decreased maximum dose with the higher normalized percentage.
Besides, the mean dose of PTV and OAR20 were also calculated and depicted in Figure 3.
For the plans normalized to 65%, 70%, 75%, 80% IDL, it is 39.08 ± 0.74, 37.53 ± 0 .56, 36.16 ± 0.35, 34.80 ± 0.25 Gy in PTV respectively, while it just increases slightly in OAR20.
Dosimetric parameters evaluation
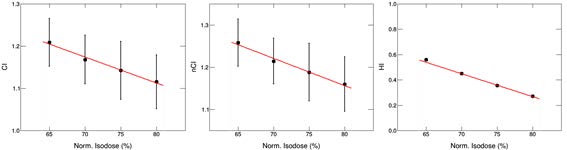
As shown in Figure 4, CI, nCI and HI all decrease with the increase of normalized IDL. Among them, as the normalization percentage increases from 65% to 80%, the CI value changes from 1.24 ± 0.01 to 1.16 ± 0.09. Likewise, the value of nCI changes from 1.28 ± 0.10 to 1.18 ± 0.09, HI changes form 0.54 ± 0.01 to 0.25 ± 0.01.
In addition, Figure 5 shows the GI variation versus tumor volume and normalized percentage. As it illustrates, for the tumor volume less than 30 cc, GI value is basically the same under different IDL, and it increases rapidly with the increase of tumor size. However, as the volume continues to increase, the value of GI is greatly affected by the change of IDL rather than volume. Furthermore, for the plans normalized to 65%, 70%, 75%, and 80% IDL, GI values are 6.89 ± 2.60, 7.17 ± 2.72, 7.33 ± 2.84, and 7.57 ± 2.95 respectively.
DISCUSSION
With the increasing application of SBRT in clinical radiotherapy, more and more attention has been paid to its dosimetric characteristics. Increasing studies have shown that the design of the treatment plan differs significantly [8,12]. Generally, with regard to CK treatment, the prescription dose always practices to an IDL ranges from 50-80% of the maximum dose inside the target [15]. As reported, it would affect the conformity, homogeneity and the dose falloff gradient of the dose distribution inside and outside of the target [13]. Currently, there are few studies refer to the normalized IDL selection in the SBRT plans of head tumor. Therefore, in this study, we examined the dosimetric difference and dose falloff characteristics of ten cases of head tumor treated by CK under different IDL selection, hoping to make a reference for clinic treatment planning.
Primarily, as depicted in Figures 1 and 2, the 2D dose distribution and the mean dose of OAR20 both reveal a fact that lower radiation energy is delivered to normal tissue in plans normalized to a lower IDL. Moreover, it also shows that lower normalized IDL results in smaller GI value, which means a steeper dose falloff between the target and adjacent healthy tissue. It is well known that the dose deposited in normal tissue is relevant to most late complications, adverse effect and secondary malignancies [12,16]. Therefore, the sharp decrease of the dose at the edge of target has gradually become a sign of the clinical optimal treatment [14,17]. The plans normalized to lower IDL just meet the requirement of clinical normal tissue protection [11,18], which can produce a steeper dose falloff outside the target and better spare OAR. However, as our study shows, the undesirable high dose area is also created in the plans with lower normalization percentage. What’s worse, the dose conformity and homogeneity in target area are inferior to the plans with higher normalized percentage as well. Hence, in clinical treatment planning, for cases that aim to relieve pain or protect important normal organs [1,17], a lower normalized percentage can be considered to achieve better clinical treatment effect.
Besides, as assessed by DVH, CI, nCI and HI [6,7], the plans normalized to a higher IDL provide better dose conformation of the prescription and greater homogeneity of dose in PTV, which avoid high dose area as well. Therefore, for cases that should confirm the dose distribution to PTV in 3D strictly, a higher normalized percentage is preferred.
Furthermore, it is comprehensible that there existed an inverse correlation between dose gradient and homogeneity [16,19]. To be specific, a sharp drop in dose means a rapid change in dose in a given area. Thus, it means inhomogeneity of dose in PTV. In our study, the prescription dose contour covered PTV uniformly in plans under different normalized percentage. Therefore, a steep dose falloff will result in a high dose area in PTV for the plans normalized to lower IDL. Therefore, the contradiction between dose gradient and homogeneity should be cautiously balanced when making treatment plans, especially for tumors with complex anatomical and physiological environments.
Ultimately, it is important to note the relationship between GI and tumor size. As shown in Figure 5, the value of GI is sensitive to the tumor volume. The correlation may be due to the fact that smaller target are more susceptible to the same dose gradient distance [16] That is to say, at the same dose gradient, the dose attenuation is proportional to the distance or volume, that is, small tumors produce small dose attenuation, while large tumors produce the opposite. However, the logic in plan making is reversed; it is to produce the same dose falloff in different tumor volumes under the same normalized percentage. So, GI increases with tumor size. Thus, the little difference made by prescription practice on small tumor can be comprehended in a similar way. Therefore, tumor volume is also an important factor to be considered in the treatment planning. Specifically, for small tumors, there is little difference in IDL selection, so the treatment plan is mainly to ensure dose coverage and homogeneity in the target area. As for large tumors, the treatment plan design needs to consider the aforementioned conditions.
To concluded, this study compare the dose distribution of different IDL normalized CK treatment plans and found the variance of dose conformity, homogeneity and dose falloff gradient against different IDL, providing reference for clinical treatment planning. In detail, the lower normalized percentage can provide better OAR protection and reduce the probability of later complications. Moreover, a higher normalized percentage can shape the dose distribution to PTV and improve PTV homogeneity. Furthermore, tumor volume is also an important factor affecting the treatment plans design that should pay more attention to. However, the study only focused on the dose differences of head tumor treatment plans under different IDL, further studies on other influencing factors and tumor sites are needed to conduct a complete comparison of the dose characteristics of SBRT treatment plans.
Declarations
Ethics approval and consent to participate.
The study was approved by the institutional review board of our hospital.
Consent for publication
The consents for publication of data have been obtained from patients.
Availability of data and materials
Not applicable.
Competing interests
The authors state that they have no competing interests.
Funding
This study was supported in part by the China Postdoctoral Science Foundation (Grant No. 2018M640725), Medical Scientific Research Foundation of Guangdong Province (Grant No. A2018020).
Acknowledgements
No acknowledgement.
REFERENCES
- Khoo VS, Oldham M, Adams EJ, Bedford JL, et al. (1999). Comparison of intensity-modulated tomotherapy with stereotactically guided conformal radiotherapy for brain tumors. Int J Radiation Oncol Biol Phys. 45(2): 415-425.
- Udrescuv C, Chapet O, Pignata B, Sotton MP, et al. (2013). The impact of the number of fields on a sbrt-imrt plan for prostate cancer. an evaluation with different objective criteria. Abstracts SFPM Ann Meeting 2013/Physica Medica. 29: E41-E42.
- Slosarek K, Osewski W, Grzadziel A, Radwan M, et al. (2014). Integral dose: Comparison between four techniques for prostate radiotherapy. Report Pract Oncol Radiother. 20(2): 99-103.
- Zhang GG, Ku LC, Dilling TJ, Stevens CW, et al. (2011). Volumetric modulated arc planning for lung stereotactic body radiotherapy using conventional and unflattened photon Beams: A dosimetric comparison with 3D technique. Radiation Oncol. 6: 152.
- Mancosu P, Clemente S, Landoni V, Ruggieri R, et al. (2016). SBRT for prostate cancer: challenges and features from a physicist prospective. Physica Medica. 32(2): 479-484.
- Kim SY and Cho JH. (2014). A dose comparison of proton radiotherapy and photon radiotherapy for pediatric brain tumor. Radiation Effec Defect Solids. 169(12): 1031-107.
- Islam MA, Yanagi T, Mizoe J, Mizuno H, Tsujii H. (2008). Comparative study of dose distribution between carbon ion radiotherapy and photon radiotherapy for head and neck tumor. Radiat Med 26(7): 415-421.
- Choi YE, Kwak J, Song SY, Choi EK, et al. (2015). Direct plan comparison of rapidarc and cyberknife for spine stereotactic body radiation therapy.
- Hossain S, Xia P, Huang K, Descovich M, et al. (2010). Dose gradient near target–normal structure interface for nonisocentric cyberknife and isocentric intensity-modulated body radiotherapy for prostate cancer. Int J Radiation Oncol Biol Phys. 78 (1): 56-63.
- Ceylan C, Kucuk N, Ayata HB, Guden M, et al. (2010). Dosimetric and physical comparison of imrt and cyberknife plans in the treatment of localized prostate cancer. Repor Pract Oncol Radiother. 15 (6): 181-9.
- Burghelea M, Verellen D, Gevaert T. Depuydt T, et al. (2014). Feasibility of using the vero sbrt system foe intracranial SRS. J Applied Clin Med Phys. 15(1): 1-8.
- Paddick I and Lippitz B. (2006). A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 105: 194-201.
- Chiu J, Pierce M, Braunstein SE, Theodosopoulos PV, et al. (2016). Sharpening peripheral dose gradient via beam number enhancement from patient head tilt for stereotactic brain radiosurgery. Phys Med Biol. 61 (20): N532-N541.
- Moran JM, Radawski J, and Fraass BA. (2005). A dose-gradient analysis tool for IMRT QA. J Applied Clin Med Phys. 6: 62-73.
- Ma L, Sahgal A, Descovich M, Cho YB, et al. (2010). Equivalence in dose fall-off for isocentric and nonisocentric intracranial treatment modalities and its impact on dose fractionation schemes. Int J Radiation Oncol Biol Phys. 76(3): 943-948.
- Ohtakara K, Hayashi S and Hoshi H. (2011). Dose gradient analyses in linac-based intracranial stereotactic radiosurgery using Paddick’s gradient index: Consideration of the optimal method for plan evaluation. J Radiat Res. 52(5): 592-599.
- Perez-Andujar A, Chang J, Hossain S, Higby C, et al. (2013). Effect of dose gradient on the incident probability of brain metastases in hippocampal-sparing whole brain irradiation. Poster Viewing Abstracts. 87(2): S757.
- Massager N, Maris C, Nissim O, Devriendt D, et al. (2009). Experimental analysis of radiation dose distribution in radiosurgery ii. dose fall-off outside the target volume. Stereotact Funct Neurosurg. 87 (3): 137-142.
- Bender ET. (2014). Increasing dose gradient and uniformity in small fields using modulation: Theory and prototypes for cone-based stereotactic radiosurgery. Med Phys. 41(5): 051706-1-7.
Copyright: Ren H, et al. © 2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.