Information Links
Related Conferences
Previous Issues Volume 9, Issue 10 - 2024
The Art and Science of Plastination: Revolutionizing Anatomy Education and Public Perception of the Human Body
Enaohwo Taniyohwo Mamerhi1, Osadjere Oghenekevwe Sonia1, Isioma Cynthia Nwaokoro2, Owhefere Great Owhefere2, Okoro Ogheneyebrorue Godswill2,*, Egwunyenga Michael Oge1 Kaine Omashim Oluwakemi1, George Kelvin Nkem2, Obie Rukevwe1
1Department of Human Anatomy and Cell Biology, Delta State University, Abraka, Nigeria
2Department of Human Anatomy, University of Delta, Agbor, Delta State, Nigeria
*Corresponding Author: Okoro Ogheneyebrorue Godswill, Department of Human Anatomy, University of Delta, Agbor, Delta State, Nigeria, Phone: 07033314640, Email: [email protected]
Received Date: November 22, 2024
Published Date: December 27, 2024
Citation: Mamerhi ET, et al. (2024). The Art and Science of Plastination: Revolutionizing Anatomy Education and Public Perception of the Human Body. Mathews J Case Rep. 9(10):194.
Copyrights: Mamerhi ET, et al. (2024).
ABSTRACT
Plastination, a groundbreaking preservation technique invented by Dr. Gunther von Hagens, has transformed the fields of anatomy and public education. By replacing bodily fluids and fats with durable polymers, plastination preserves biological specimens in intricate detail, enabling detailed study and long-term exhibition. This review explores the dual role of plastination as a scientific tool and a medium for public engagement. It examines its profound impact on medical education, offering unparalleled opportunities for hands-on learning and anatomical accuracy. Additionally, the paper delves into the success of public exhibits like Body Worlds, which have sparked global interest and dialogue about the human body, health, and mortality. Ethical considerations, including consent and cultural sensitivities, are also critically analyzed. By combining scientific rigor with artistic presentation, plastination not only revolutionizes anatomical studies but also reshapes societal perceptions of the human form and mortality. This review highlights the enduring significance of plastination in bridging the gap between science and society.
Keywords: Plastination, Anatomy Education, Body Worlds, Ethical Consideration.
INTRODUCTION
Plastination is a method or process used in anatomy whereby body parts or bodies are preserved. It is a process in which human and animal tissues are replaced with synthetic materials such as resins, silicon or epoxy polymers [1]. Plastination is a preservative process used to generate non-toxic anatomical specimen which are used for a long period for instructional and educational purposes. These plastinates are odourless, dry, resistant to decay and lasting which are helpful tools not only to the medical students but also to the public [2-5]. This technique has become widely used in medical education, museum exhibits and research laboratories around the world [6].
Plastination is a preservative technique used to prepare biological specimen for study or display. It involves replacing the water and fat in tissue with curable polymers of which these specimens retain their original structure and appearance allowing for detailed examination and long term preservation [7]. Plastination offers several advantages over traditional methods of which these specimens can be dissected without the need of specialised equipment making them valuable tools for teaching and research in various fields of study [8].
One of the key benefits of plastination is the preservation of fine anatomical details allowing the creation of anatomical specimens that retain their natural appearance and flexibility [9]. Plastinated specimens are mostly used in medical schools and universities for teaching purposes as they provide hands-on learning experience without the need of constant preservative measures [10]. This review highlights the enduring significance of plastination in bridging the gap between science and society.
History of Plastination
Plastination is a revolutionary technique that has changed and improves the way we study human anatomy. Gunther Von Hagens a German anatomist, lecturer and a businessman developed the concept of plastination; his technique was invented and patented between 1977 and 1982; his initial experiments involved using epoxy resin to replace the water and fat in tissues, creating durable and lifelike specimens [11].
Von Hagens was born on January 10th 1945 in Gunter Gerhard Liebchen in Ait-Skalden now called Skalmierzyce near Ostrowo Reichsgau Wartheland in German-annexed Poland. Von Hagens parents took him away when he was five days old on a six month trek westwards running away from the advancing red army and the imminent soviet occupation. Von Hagens as a child was haemophiliac which caused him to spend six months in the hospital after being injured, this intrigued his desire in the medical field and in 1965 he started studying medicine at the University of Jena [12].
Source: Science Photo Library
The institutes of anatomy and pathology at the university of Heidelberg appointed Von Hagen as a lecturer and founded the institute of plastination in 1993, in 1996 he became a visiting professor and opened a second institute of plastination at the university of Dalian China, Von also directs a plastination center at the state medical academy in Bishkek Kyrgyzstan also a guest professor at the New York university college of dentistry in the year 2004. In the year 1980s and 1990s plastination gained popularity as a valuable tool in medical exhibition, anatomical exhibition featuring plastinated specimens became popular attractions in museums and science centres showcasing the intricate structures of the human body in a way that was both educational and visually compelling [13].
Types of Plastination
There are several types of plastination based on the purpose and presentation of the specimen, each type of plastination serves specific purpose in education research and scientific exploration which provide valuable insights into anatomy, pathology and the natural world [13]. These include:
- Whole body plastination
- Organ plastination
- Slice plastination
- Transparent plastination
- Pathological plastination
- Comparative plastination
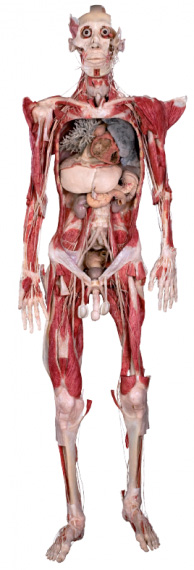
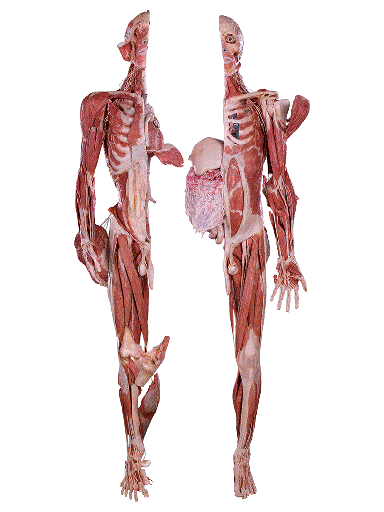
Whole Body Plastination: This is a comprehensive technique that involves preserving an entire human or animal body in a lifelike and anatomically accurate state. It is often used in medical education, research and museum exhibits to study the entire anatomy of organisms [13]. The process of whole body plastination begins with selection of suitable donor body or specimen, the body undergoes thorough cleaning and preparation including removal of internal organs and tissues that are not part of the plastination process. Fixation is carried out using formaldehyde solution to prevent decay and maintain tissue structure. The specimen is dehydrated for the removal of water and lipids using solvents like acetone, the dehydrated body is then immersed in a bath containing polymerisable monomer such as silicon rubber or epoxy resin, then polymerisation occurs by solidifying and creating a durable lifelike plastinated body [14].
Source: Science Photo Library
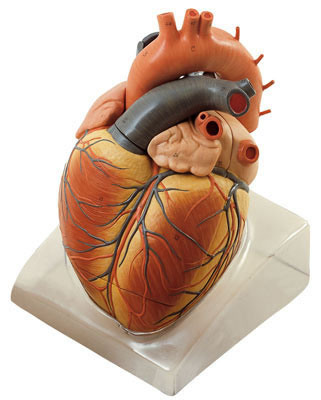
Organ Plastination: It focuses on preserving individual organs such as heart, lungs, brain, liver and kidneys. These plastinated organs are valuable for studying organ structure, function and pathology. The specific organs are selected and fixed using formaldehyde or any suitable fixative agents to prevent decay and maintaining of organ structure, dehydration is carried out using dehydrating agent like acetone to remove water and lipids from the organ. The dehydrated organ is immersed in silicon rubber or epoxy resin or any polymerisable monomers which solidifies to create a plastinated organ. Polymerisation may involve heat, ultraviolet light or chemical catalysts to ensure the polymer’s curing and hardening.
Source: Scientific Diagram
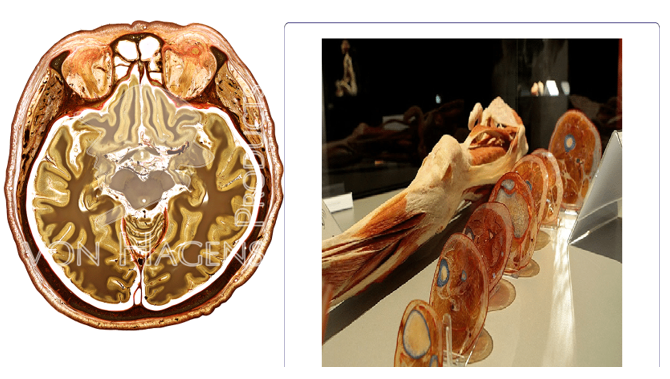
Slice Plastination: In slice plastination also known as sheet plastination, specimens are sliced into thin section (slices or sheets) which are plastinated and arrange in sequence. This type of plastinate allows for the creation of three-dimensional representations of anatomical structures such as organs, systems or specific body region. The process for slice plastination begins with specimen fixation using a formaldehyde solution or any suitable fixative agent, then the specimen is sliced into thin section or sheets using a microtome or a vibratome; the thin section typically ranges from millimeters to micrometers in thickness and are dehydrated using acetone to remove water and lipids [15]. Each dehydrated section is then plastinated using polymerisable polymer which will then solidify to create a plastinated slice. These plastinated slices are arranged in sequence either flat or stacked in order to reconstruct the original anatomical structure in a three-dimensional pattern. Slice plastinates are used to study complex anatomical structures such as organ systems, nerve pathways and tissue layers by giving comprehensive view of internal structures.
Source: Scientific Diagram
Transparent Plastination: It is a specialised technique that involves impregnating specimens with transparent polymers such as acrylic resins; the plastinates are clear and see-through allowing light to pass through for a clearer visualization of internal structures [13].
Pathological Plastination: It focuses on preserving diseased or pathological tissues and organs. Theses plastinated specimens are used to study disease processes, abnormalities and medical conditions aiding in diagnosis research and education.
Comparative Plastination: It involves plastinating specimens from different race in humans, species of plant and animals for a comparative anatomy studies. This type of plastinates helps researchers and educators understand anatomical similarities and differences across races and species contributing to evolutionary studies and biological research [16].
Plastination Techniques
There are several types of plastination techniques commonly used in anatomical preservation. Each technique has its advantages and is chosen based on factors such as specimen size, desired transparency, flexibility and intended use (e.g. teaching, research or museum display) [17]. These include:
- Silicon plastination
- Epozy plastination
- Polymerization plastination
- Acrylic plastination
- Sheet plastination
- Silicon Plastination: This is one of the most widely used plastination techniques in anatomical preservation due to its versatility and effectiveness in producing high quality specimens. It involves impregnating specimens with liquid silicone rubber which is then cured to create a solid and durable specimen. Silicon plastination preserves the natural colour, texture and flexibility of tissues, making it suitable for detailed anatomical study and long-term display. Silicone is also transparent allowing for visualisation, they are durable, resistant to decay and can be handled without the risk of deterioration [17].
- Epoxy Plastination: This involves replacing water and fat in tissues with epoxy resin which will hardens to create a rigid and durable specimen. It is often used for larger specimens or those requiring a high level of structural support. Epoxy resin can be tinted to enhance contrast and highlight specific structures within the specimen. One disadvantage of epoxy resin is that it lacks flexibility and transparency; epoxy plastinated specimens are used in medical education, research, museum exhibits, demonstrating of surgical procedures and showcasing of pathological conditions.
- Polymerisation Plastination: In this technique, specimens are impregnated with a polymerisable monomer such as polyester resin and then the specimens are polymerized using heat, ultraviolet light or chemicals. Polymerisation plastination produces specimen with excellent detail and durability. Polymerisation can also produce transparent specimen by using clear polymer matrices which allows for the visualization of internal structures.
- Acrylic Plastination: It uses acrylic polymers to preserve specimens, this technique can produce transparent specimens that are suitable for teaching and research purposes allowing for visualization of internal structures. Acrylic plastinates retain their natural colour and textures providing a lifelike appearance, they are durable and resistant to decay making them suitable for long-term storage and display.
- Sheet Plastination: This involves slicing of specimens into thin sections, dehydrated and impregnated with resin. The section is then stacked and cured to create a three dimensional representation of the specimen’s internal structures. Thicker sections may be used to study gross anatomical features and organs while thinner sections are suitable for examining cellular structures [18].
Types of Polymers used in Plastination
There are several types of polymers used in plastination, each with specific properties and application in preserving biological tissues for anatomical study and research. Below are some commonly used types of polymers in plastination:
- Silicone rubber
- Epoxy resins
- Polyester resins
- Acrylic polymers
- Polyurethane resins
- Hydrogel polymers
Silicon polymers particularly polydimethysiloxane (PDMS) are widely used in plastination due to their excellent properties which makes them well-suited for preserving and displaying of anatomical specimens. Silicone polymers are optically clear allowing for exceptional visualisation of internal anatomical structures in plastinated specimens. PDMS based silicon rubber retains a degree of flexibility even after curing making it suitable for plastinating specimens that require articulation or dynamic displays which allows for the creation of lifelike poses and functional joint movements; silicone polymers also exhibit elasticity enabling them to withstand mechanical stress and deformation without losing their structural integrity, contributing to the durability and longevity of plastinated specimens [19]. Silicone polymers used in plastination are typically formulated to be non-toxic and biocompatible ensuring safety for handling, display and educational use. Silicon polymers penetrate tissues effectively by filling intercellular space and coating cellular structures during impregnation. Silicone polymers can be tinted to enhance visibility of specific anatomical features within plastinated specimens. Silicon polymers exhibit chemical stability, resisting degradation, discolouration or changes in physical properties over time; this stability ensures long term preservation and display of plastinated specimens. Silicon polymers are inherently hydrophobic, repelling water and moisture which contributes to the long term stability and preservation of plastinated specimens by preventing water-induced degradation, microbial growth and mold formation.
Epoxy resins are widely used in plastination for their excellent preservation properties, durability and versatility in creating rigid and detailed anatomical specimens. Epoxy create a rigid and durable polymer matrix within tissues by providing structural support and stability to plastinated specimens by ensuring plastinated specimens maintain their shape, size and anatomical features without deformation or shrinkage over time. The strong bonding and cross-linking properties of epoxy resins preserve the integrity of tissue structures including organ morphology, vascular networks and cellular arrangements. Epoxy resins impregnates tissues effectively by penetrating tissues deeply reaching fine anatomical structures such as nerves, blood vessels and micro-anatomical features, this penetration depth allows for the preservation and visualisation of intricate details in plastinated specimens. Epoxy resins can be sculpted or shaped after impregnation and curing for aesthetic purposes also epoxy resin surfaces can be textured or finished to mimic natural anatomical textures such as organ surfaces, muscle fibres or bone structure. Epoxy resin adheres well to biological tissues and substrates forming bonds that prevent separation of the polymer matrix from tissue structures ensuring long term stability and durability of plastinated specimens. Epoxy resins can be coloured to enhance visibility of specific anatomical features while some epoxy resins are formulated to be transparent allowing for clear visualisation of internal anatomical structures in plastinated specimens, radio-opaque additives can be incorporated into epoxy resins allowing plastinated specimens to be visualised and study using imaging techniques like x-rays, CT scan and MRI. Epoxy resins exhibit chemical stability resisting degradation, discolouration or changes in the physical properties over time. Some epoxy resins formulations offer ultra-violet protection preventing photo-degradation and colour fading in plastinated specimens that are exposed to light or outdoor display. epoxy resins are suitable for plastinating whole body specimens, organ systems (cardiovascular system, respiratory system and nervous system) allowing for focused anatomical study and comparison; epoxy resins are also effective in plastinating tissue sections, histological specimens, and anatomical slices providing detailed insights into cellular structures and tissue organisation.
Polyester resins such as polyethylene glycol (PEG) are utilized in plastination of specimens due to their unique properties that contributes to effective tissue preservation and anatomical representation. Polyester resins like PEG are flexible and soft making them suitable for plastinating delicate tissues, organs and anatomical structures that requires resilience and flexibility allowing plastinated specimens to retain their shape and structural integrity even when subjected to bending, stretching or manipulation. Polyester resins can penetrate deeply filling interstitial spaces and coating cellular structures during impregnation and ensures preservations of tissue structures. PEG has hydrating properties retaining moisture within plastinated specimens and mimicking the natural hydration state of tissues by preserving tissue elasticity, texture and pliability; they are also good in plastinating soft tissues like muscles, nerves, adipose tissue and mucosal membranes maintaining their soft and supple characteristics. Polyester resins used in plastination are typically formulated to be biocompatible and non-toxic ensuring safety for handling, display and educational use. Polyester resins are compatible with various staining techniques used in histology and anatomical research to enhance contrast and highlight in specific anatomical details. PEG based polyester resins may exhibit temperature sensitivity during handling and processing therefore a controlled temperature environment should be maintained to optimised the plastinated specimens; also polyester resins being water soluble requires careful handling to prevent exposure to moisture which could affects the plastinated specimen [20].
Acrylic polymers are utilised in plastination for their transparency, hardness and versatility in creating detailed and durable anatomical specimens. Acrylic polymers such as polymethyl-methacrylate (PMMA) offers high transparency allowing for clear visualisation of internal anatomical structures in plastinated specimens also they have minimal optical distortion or refraction ensuring accurate representation of tissue features. Acrylic polymers create a rigid and hard polymer matrix within tissues by providing structural support and stability by maintaining shape, size and anatomical features plastinated specimens without deformation. Acrylic polymers impregnate tissues effectively filling intercellular spaces and coating cellular structures also deeply reaching fine anatomical structures such as nerves, blood vessels and micro-anatomical features which allows for the preservation and visualisation of intricate details in plastinated specimens. Acrylic polymers can be tinted and pigmented to enhance visibility of specific anatomical features; they can also be polished to mimic natural anatomical textures such as organs surfaces, muscle fibres and bone structures which add realism to plastinated specimens. Acrylic polymers exhibit chemical stability by resisting degradation, colouration or changes in the physical properties which ensures the long-term preservation and display of plastinated specimens; also some acrylic polymers are formulated with ultraviolet protections preventing photo-degradation and colour fading in plastinated specimens that are exposed to light or outdoor display. Acrylic polymers are suitable for whole body plastination, organ systems (cardiovascular system, respiratory system and nervous system), tissue sections, histological specimens and anatomical slices allowing for detailed insights into cellular structures and tissue organization [21].
Basic Principle and Methodology of Plastination
The principles and methodology in plastination are based on several key principles and steps that ensure the successful preservation of biological specimens [22].
The principle of plastination includes:
- Anatomical Accuracy: The primary principle of plastination is to preserve with utmost anatomical accuracy. This involves maintaining size, shape, colour and texture of tissues and organs during plastination process. Careful fixation, dehydration and impregnation techniques are employed to ensure that the plastinated specimens accurately represent the original anatomical structures.
- Versatility: Plastination is a versatile technique that can be applied to a wide range of biological specimens such as human tissues, animal organs and plants. This versatility allows for the creation of diverse plastinated specimens for educational, research and artistic purposes.
- Educational Value and Research Support: Plastinated specimens serve as valuable tools for teaching anatomy, pathology, surgical techniques and medical procedures. They provide hands on learning experiences, promote interactive learning environments and facilitate the understanding of complex anatomical structures. It also serves for anatomical research by enabling detailed examination of tissues, organ systems, developmental stages and pathological conditions. It contributes to scientific discoveries, medical advancement, anthropology and paleontology.
- Ethical Consideration: Ethical considerations are integral to plastination practices especially when working with human donor tissues. Respect for donor consent, confidentiality, cultural sensitivity and ethical guidelines are paramount and are upheld throughout the plastination process.
- Structural Integrity and Minimization of Artefacts: Plastination aims to preserve the structural integrity of biological specimens including tissues, organs and anatomical structures, this principle ensure that the plastinated specimens maintain their natural form, arrangement and connectivity of anatomical components. It also seeks to minimize artefacts or distortions that may occur during the preservation process, careful handling of equipments, precise techniques and quality control measures are implemented to reduce artefacts and maintain the accuracy of plastinated specimens.
- Quality Assurance and Environmental Sustainability: Plastinated principles emphasize quality assurance throughout the process that is from specimen selection to post-processing, quality; control measures and standards are implemented to ensure consistency, reliability and reproducibility of plastinated specimens. Consideration for environmental sustainability and conservation is also considered of which effort should be made to minimize environmental impact by using eco-friendly materials and practices.
- Functional Preservation and Histological Detail: Plastination focuses on preserving functional aspects of tissues and organs by maintain tissue flexibility, elasticity and mechanical properties to stimulate natural physiological conditions. It also strives to retain histological details within tissues such as cell morphology, arrangement and tissue architect. This level detail allows for microscopic examination and study of cellular structures.
- Longetivity and Non-Toxicity: Plastinated specimens are designed to be durable, stable and long lasting; they should withstand environmental factors, storage conditions without deteriorating, shrinkage or deformation. It should also prioritise non-toxicity and safety for both users and the environment this ensures that plastination practices are environmentally friendly and compliant with health standards.
METHODOLOGY
- Specimen Selection and Preparation: Specimens are carefully selected based on the desired outcome and application of the selected specimen. Plastination process begins with specimen preparation by selecting appropriate specimens based on either educational, research or artistic objectives; these specimens are carefully handled, cleaned and trimmed to remove excess tissue or debris before proceeding.
- Fixation and Dehydration: This is a crucial step in plastination which preserve the structure and cellular integrity of specimens; use of fixative agent like formaldehyde to preserve tissue structure and tissue degradation, the duration and type of fixation depends on the specimen size, type and intended use. During dehydration water and lipids are removed from the specimen using alcohol to prepare specimen for impregnation; the specimen is immersed in a series of graded alcohol solution.
- Impregnation: Once dehydrated, the specimen undergoes impregnation with poly-merisable monomers such as silicon rubber, epoxy resin and acrylic polymers. The impregnation step ensures thorough penetration of the monomer into the tissue by replacing the solvent molecules and filling interstitial spaces within the specimen.
- Vacuum or Pressure: During impregnation, vacuum or pressure technique may be employed to enhance monomer penetration and distribution within the tissue. Vacuum cycles remove air bubbles and facilitate monomer infiltration while pressure cycles promote deeper penetration of monomer into dense and complex anatomical structures.
- Polymerisation: This is the process of converting the impregnated monomers into solid polymers which will result to the plastination of the specimen. Polymerisation is achieved through various methods such as heat curing, chemical catalysts, ultraviolet light and or depending on the type of monomer used.
- Curing and Hardening: After polymerisation, the plastinated specimen undergoes curing and hardening to ensure structural stability and durability. This may involve additional treatment like cooling, rinsing or trimming of excess polymer to refine the final appearance of the plastinated specimen.
- Staining and Contrast Enhancement: Though this is optional but in some cases, plastinated specimens may undergo staining or coloring to enhance visual contrast and highlight specific anatomical features. Staining techniques may include dyes, contrast agents, or specialized solutions to differentiate pathological tissues or simulate pathological conditions for educational purposes.
- Mounting and Display: Once fully plastinated and post-processed, specimen is mounted or displayed according to their intended use. They may be embedded in transparent blocks, arranged in anatomical sequences or integrated into educational models and exhibits which could be used for teaching research or public display.
- Documentation and Quality Control: Throughout the plastination process, document-tation and quality control measures are essential; detailed records of specimen handling, processing steps, material used and quality assessments are maintained to ensure consistency, reproducibility and quality assurance in plastinated specimens. This helps to identify any imperfections, artifacts or inconsistency that may require corrective measures.
- Embedding in Transparent Resins: For certain application like slice and transparent plastination, plastinated specimens are embedded in transparent resin blocks or matrices. Embedding enhances the durability of specimens and allows for three-dimensional visualization and provides protection against external factors such as moisture and humidity, chemical exposure ultraviolet radiation, biological contaminants light exposure and temperature fluctuations.
- Sectioning Technique: In techniques such as slice or sheet plastination, precise sectioning of specimens is essential, this may involve using freezing microtomes, vibratomes or any other cutting instruments to obtain uniform and thin section for impregnation and polymerization. The thickness of sections is carefully controlled based on the desired outcome and specimen type.
- Decompression and Refilling: In techniques like whole body plastination, decom-pression and refilling steps are used to remove air from the specimen’s internal cavities and replace the air with polymerisable monomers. This ensures thorough impregnation and preservation of internal structures such as blood vessels and organs in the plastinated specimen. Posterior filling methods fills hollow organs such as lungs and intestines with monomers while displacement method using weight bags or fixtures helps in maintain of proper anatomical positioning preventing collapse during impregnation and polymerisation.
- Cryo Plastination: This is a variation of plastination that involves freezing specimens before impregnating with monomers, this technique helps in preserving tissue properties such as the elasticity and moisture content and it is particularly suitable for specimens requiring flexibility.
CONCLUSION
In conclusion, plastination stands as a revolutionary technique in anatomical preservation, offering unparalleled advantages in education, research and medical practice. Through its various methods like silicon plastination, epoxy plastination and more, it provides durable, odourless and accurate anatomical specimens that are invaluable in teaching and understanding human anatomy coupled with stains and immunohistochemistry, plastination enhances our insight into neuroanatomy aiding in the exploration and comprehension of complex neural structures. Stains enhance the educational and research value of plastinated specimens facilitating deeper understanding of physiological processes and pathological conditions.
Looking ahead, the future of plastination appears promising with ongoing advancements in preservation methodologies and imaging technologies, these developments pave the way for interactive virtual anatomy platforms, personalized medical education tools and novel research avenues that leverage the benefit of plastination while embracing modern innovations.
In essence, plastination transcends traditional anatomical preservation methods, embodying a fusion of art and science that continues to inspire curiosity, discovery and excellence in the study of human anatomy and pathology.
REFERENCES
- Smith J. and Johnson PH. (2018). Defining plasination: a revolutionary techniques for anatomical preservation. Journal of Medical Science and Research. 29(3):198-203.
- Weiglein AH, Schaller F. (2019). New insights into plastination techniques: A comprehensive review. Journal of Anatomy. 234(5):699-711.
- Moke EG, Ekuerhare B, Enaohwo MT, Asiwe JN, Ofulue OO, Umukoro EK, et al. (2022). Resistant hypertension. Journal of Drug Delivery and Therapeutics. 12(3):230-235.
- Omogbiya AI, Moke EG, Ojieh AE, Enaohwo, MT, Umukoro EK, Anachuna KK, et al. (2020). Evaluation of perception, attitude, and impact of club-house noise pollution on mental health of individuals living within proximity of club-houses in Abraka, Delta State, Nigeria. Journal of Applied sciences and environmental management. 24(6):1009-1013.
- Enaowho TM, Okoro OG. (2018). Anthropometric study of the frontal sinus on plain radiographs in Delta State University Teaching Hospital. Journal of Experimental and Clinical Anatomy. 17(2):49-52.
- Udi OA, Okoro OG, Enaowho TM, Douglas B. (2023). Nutritional knowledge and body mass index among students at Novena University, Ogume, Nigeria. Folia Medica Indonesiana. 59(1):20-25.
- Brown M, Liu X. (2020). Preservation of neural pathways through neuroplastination: techniques and applications. Journal of Neuroscience Methods. 335:108632.
- Wilson D, Gupta A. (2020). Combining plastination with immunohistochemistry: A novel approach to studying the brain. Brain Research Bulletin. 155(3):45-54.
- Chen Y, Wong M. (2020). Improved visualization of cellular structures in plastinated tissues using combined histological and immunohistchemical staining. Journal of Histotechnology. 43(3):214-223.
- Taylor H, Roberts L. (2020). Ethical considerations in the use of plastinated specimens in education and research. Journal of Medical Ethics. 46(7):452-458.
- Von HG, Tiedemann K. (2020). Plastination: definition, historical background and technical process. Anatomical Sciences Education. 13(2):145-154.
- Miller RJ, Ahmed N. (2018). A comprehensive review of plastination: historical background and Technical processes. Journal of Anatomical Sciences. 46(2):95-106.
- Schiller H, Panke J. (2019). Overview of plastination techniques: from standard to advance methods. Journal of Anatomy. 234(6):912-920.
- Lee J, Smith K. (2019). Nissl staining in plastination: techniques and applications. Journal of Histotechnology. 42(3):184-195.
- Chavez L, Martinez R. (2023). Long-term storage solutions for plastinated specimen in research facilities. Journal of Biological Preservation. 29(2):144-158.
- Johnson DH, Peters C. (2018). The application of plastination in pathological studies and specimen preservation. Pathology Today. 34940:210-212.
- Rivera S. and Marshall JR. (2019). Technological advancement in plastination and their implications for anatomical studies. Journal of Anatomy and Physiology. 58(4):312-325.
- Nguyen T, Pham L. (2020). Van Gieson staining in plastination: techniques and effectiveness. Journal of Histotechnology. 43(4):290-303.
- Smith J, Zhao L. (2020). Silicon plastination: variants and applications. Histology and Histopathology. 35(1):21-33.
- Gonzalez P, Ortiz F. (2023). Advancements in plastination technology: from traditional methods to modern innovations. Biomedical Advances. 37(2):145-159.
- Brown MA, Johnson PA. (2021). Fundamentals of plastination: Understanding the science behind the process. Journal of Medical Science and Research. 29(3):198-209.
- Hassan M, Amed R. (2022). Utilizing plastination for long term preservation of biological specimens in research settings. Journal of Research Methods. 48(2):89-103.
.png)