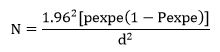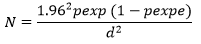Previous Issues Volume 9, Issue 1 - 2025
Seroprevalence of Salmonellosis in Intensive Poultry Farms and Associated Risk Factors in Wolaita Sodo Town, Southern Ethiopia
Gelan Dule Dahesa*, Nasir Dibo Lenjiso
School of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
*Corresponding author: Gelan Dule Dahesa, School of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia, Phone: +251982607591; E-mail: [email protected]
Received Date: December 30, 2024
Published Date: January 22, 2025
Citation: Dahesa GD, et al. (2025). Seroprevalence of Salmonellosis in Intensive Poultry Farms and Associated Risk Factors in Wolaita Sodo Town, Southern Ethiopia. Mathews J Vet Sci. 9(1):60.
Copyrights: Dahesa GD, et al. © (2025).
ABSTRACT
Salmonellosis is a significant foodborne disease worldwide, with poultry and poultry products being major sources of infection. A cross-sectional study was conducted from October 2022 to May 2023 at Wolaita Sodo town, Southern Ethiopia with the objectives of determining seroprevalence of salmonellosis, and identifying its associated risk factors. The sample size was determined based on an expected prevalence of 50% and a confidence level of 95%, because of the absence of a previous study on the seroprevalence of salmonellosis in the study area. The farm was purposively selected according to the potential of poultry production, availability of chickens and the willingness of the owner take samples from his birds and simple random sampling was used to select chickens. Methodology includes serological testing and a structured questionnaire to farm owners to gather information was used. The serum slide agglutination test was performed on the sample collected from the farm, which were subsequently organized, coded, and entered into an Excel spreadsheet. These data were then subjected to analysis using STATA. Descriptive statistics was used to summarize the data and compute prevalence. The Chi-square test was used to determine the association between the occurrence of salmonellosis and the variables. Out of 384 collected samples, 30.7% were positive for Salmonellosis. A statistical analysis revealed a significant association between infection rates and factors such as age, feed type, and farm size of the poultry has a significant association (p<0.05), but the housing system has no significant association (p> 0.05). Salmonellosis was most commonly prevalent in farm with large flock, at 65%, followed by medium farm 30.9% and small scale 6.3%. In conclusion the salmonellosis in study area was moderate. Should be invest in research and development activities to understand the factors contributing to the prevalence of salmonellosis in poultry farms in the study area. This can include studying the impact of feed types, farm sizes, and management systems on disease prevalence and identifying effective control measures.
Keywords: Intensive Poultry Farms, Risk Factors, Salmonellosis, Seroprevalence.
INTRODUCTION
The worldwide poultry population is estimated to be 16.2 billion people, with 71.6 percent living in developing countries, producing 6.7 million tons of chicken meat and 5.8 million tons of hen eggs [1]. Poultry, particularly chickens, is the world’s most extensively kept and numerous livestock species [2,3]. Village poultry accounts for more than 70% of poultry products and 20% of animal protein consumption in Africa. Ethiopia owns 42,915,629 poultry, of which 95.37 % is under rural holdings while the rest 4.63% is urban. About 98% of the total national poultry population consists of indigenous chickens and the remaining 2% consists of exotic breeds [4].
The country needs to increase the number of its poultry and intensify its management to satisfy the protein need of its ever-increasing human population estimated at 79,086,894 in 2007 [5]. The poultry population in Ethiopia is not growing and a gradual declining [6], and is highly constrained by diseases among other factors. The current rates of mortality due to diseases from day-old to adult chicken are estimated to be 20 to 50% in Ethiopia [7].
The poultry industry is facing great setbacks due to frequent outbreaks of salmonellosis. Although many developed countries have eradicated these diseases from commercial poultry, it has increased incidence in most developing countries. Fowl typhoid caused by Salmonella enterica subspecies. Enteric serovar Gallinarum biovar Gallinarum (Salmonella gallinarum), produces high mortality rates (up to 90%) in birds of all ages, thus causing heavy economic loss [8].
Salmonellosis is now a worldwide problem. Salmonella is Gram-negative a bacterium with rod shape structure, motile that best grows at 37°C specifically via water and food transmitted by the fecal-oral route. Salmonellosis is one of the most important zoonotic diseases, capable of surviving in different environments, including as spores and in an aerobic conditions. Salmonellosis is a disease characterized by treating patients with diarrhoeal and systemic symptoms [9].
Humans and animals Poultry get infected by Salmonella serovars are affected by Salmonella infection due to Salmonella among which S. Typhimurium, S. Enteritidis and S.enterica, and Salmonella bongori. Salmonella is Heidelberg and is known to infect many hosts. But, commonly found in the surrounding and any material Salmonella Enteritidis, Salmonella Kentucky, contaminated by feces of animals. Contaminated Salmonella Heidelberg is serovars most commonly sewage and effluents can be a source of infection [10].
They biochemically produce carbon dioxide and hydrogen gases from D-glucose, and typically hydrogen sulfide is produced by most Salmonellae species. All Salmonellae are atherogenic except for Salmonella serovar Typhi which never produces gas and does not produce urease, oxidase, and indole [11].
The main reservoir for Salmonellae is contaminated poultry and eggs [12]. The natural habitat of Salmonella may be divided into three categories: highly adapted to men and agents of typhoid fever; highly adapted to animals responsible for animal paratyphoid; and most of the serovars that affect men and animals [13]. The disease in chickens is characterized by significant economic losses in the poultry industry due to high morbidity, mortality especially in young chicks, and reduced production [14].
In poor nations, poultry production has significant economic, social, nutritional, and cultural benefits [15]. They are providing high biological value animal protein in the form of eggs and meat [16]. Fowl typhoid caused by Salmonella gallinarum is recognized worldwide as a disease of social and economic significance [17]. It is a septicaemic disease that affects primarily chickens and turkeys, although natural infections in ducks, pheasants, guinea fowls, peafowl, grouse, ostriches, wood pigeons, swans, sparrows, peacocks, and quail have been reported [16].
Although S. gallinarum infection is frequently considered a problem of adult and grower chickens, chicks are also affected [18]. Efforts at controlling this agent through the application of a coordinated policy of hygienic measures, together with serological testing and slaughter of positive reactors, have led to the eradication of S. gallinarum in many developed countries [15].
Although the above pilot studies were conducted on the prevalence of Salmonella in modern intensive 123 poultry farms in different urban areas of Ethiopia. So, there was no study of seroprevalence of salmonella in the study area, and due to important disease is zoonotically and contagiously cause serious problem in chickens and human beings so, it is a must to know what is the status of salmonella disease in wait for Sodo Town So to overcome such prevalence in the country or region including study area by the identification and characterization of the bacteria associated with the risk factors for the occurrence. One potential area for research that requires further investigation is the limited availability of comprehensive seroprevalence data pertaining to salmonellosis in intensive poultry farms within a specific geographical region. This study aims to bridge these research gap by determine the seroprevalence of salmonellosis in poultry farms in Wolaita Sodo town, Southern Ethiopia and investigation of associated the risk factors. The specific objectives of the study area was:
Therefore, the objectives of the study were:
- To determine the seroprevalence of salmonellosis caused by Salmonella species,
- To identify and analyze risk factors contributing the prevalence of the disease in poultry farms in Wolaita Sodo town, Southern Ethiopia.
MATERIALS AND METHOD
Description of the Study Area
The study was conducted in Wolaita Sodo town of Wolaita zone, Southern Ethiopia from October 2022 to April 2023. Sodo City is located 390 km south and 167 km southwest of Addis Ababa and Hawassa respectively. Astronomically the city is located 6049” N latitude and 37045’’ E longitude. The total area of the city is about 3,200 hectares. The city is divided into three sub-city, 11 kebeles, and 99 villages. The city is established at the foot of Mount Damot and from these mountains; its altitude descends to the south direction. The highest and lowest altitude of the town range from 2,222-1,600 meters above sea level. The mean annual temperature of the town is 20 degrees Celsius and the mean annual rainfall is 1,200mm. The chickens used in the study were raised in small-scale poultry breeding farms that raised commercial exotic chickens [10].
Figure 1. Map of Wolaita Sodo town, SNNPR, Ethiopia Source: Wolaita zone administrative and publication.
Study Population
The study populations were exotic breeds found in intensive farm systems in study area. During sampling age was categorized into three groups grower (Up to 8 weeks), pullets (8-22 weeks), and layers (above 23 weeks up to 72 weeks) based on data recored from the farm [10], fed type (Locally and Comercially), origin of farm was named as (Farm1, 2,3) because of personal security for that farm and housing (Cage and litter) system [7] were recorded.
Study Design
A cross-sectional study was conducted within the lactating dairy cows were selected using a simple random sampling method and it was conducted from October 2022 to May 2023 in Wolaita Sodo town to estimate prevalence of salmonellosis and identify its associated risk.
Sample Size Determination and Sampling Method
The study area was chosen based on past poultry production history relating to breeding and accessibility. The expected seroprevalence in the study area was assumed to be 50% at a 95% confidence interval, because of the absence of a previous study on the seroprevalence of salmonellosis in the study area. Therefore, the sample size was calculated based on the formula given by [19].
Where N= sample size
P= expected prevalence
D= desired absolute precision
The calculated sample size was 384. The farm was purposively selected according to the potential of poultry production, availability of chickens and the willingness of the owner take samples from his birds. Within each farm, Simple random sampling was used to select chickens.
Methodology
Data collection method
A structured questionnaire containing close-ended questions was used to collect data regarding the different potential risk factors for salmonellosis such as age, fed type, origin of farm and housing system by a face to face questionnaire survey farm owners/Manager to determine their association with the occurrence of salmonellosis.
Blood sample collection, transportation, storage and laboratory finding
The samples were collected in the hygiene, or aseptically and a blood samples were collected from the wing vein to prepare serum for seroprevalence [10]. Approximately 3ml of blood sample was collected from the wing vein from each bird, allowing for clotting in the syringe and the blood in the plane vacutainer tube was put in a slant position at room temperature. The plane vacutainer tube was then appropriately sealed and labeled with waterproof markers to identify the brid from which blood was collected. Samples were placed in ice boxes and transported immediately to the Microbiology Laboratory of the School of Veterinary Medicine, Wolita Sod University.
After 12 hrs of clotting, the sera were subjected to centrifugation at 1000 rpm for 10 minutes. Finally, clarified sera were stored in deep freeze until it was tested. The serum slide agglutination test was performed following the test procedure described [11]. All harvested sera and the reagent were brought to room temperature (22 ± 5°C) before use. 30 µl of crystal violet stained Salmonella Gallinarum antigens and an equal volume of the serum were added to the sterile slide. Thereafter, the two contents were mixed gently by rocking and rotating them for about 2-3 minutes [10]. then, the presence or absence of agglutination reactions was checked. Any level of agglutination is considered positive. Finally, the result was recorded.
Data Management and Analysis
The entire data set was cleaned, entered into a Microsoft Office Excel spreadsheet, and then subjected to STATA 14 analysis. Descriptive statistics was used to summarize the data and compute prevalence (the prevalence was computed by dividing the number of salmonellosis-positive birds by the total number of examined birds and multiplying by 100). The Chi-square test was used to determine the association between the occurrence of salmonellosis and the variables. P-Values < 0.05 was considered as statistically significant.
RESULTS
Prevalence Study
Out of 384 Samples, 30.7% tested positive for salmonellosis (Table 1).
Table 1. Overall prevalence of salmonellosis
|
No |
No of examined |
No of postive |
Prevalence |
|
1 |
384 |
118 |
30.7% |
Table 2. Prevalence of salmonellosis in origins of poultry farm
|
Farm |
No.Examined |
No. of Positive |
Percent (%) |
|
Farm 1 |
200 |
55 |
38.5 |
|
Farm 2 |
130 |
50 |
38.5 |
|
Farm 3 |
54 |
13 |
24.1 |
|
Total |
384 |
118 |
30.7 |
The p-value ˂ 0.05 indicates that there is a statistically significant association between the age of poultry and the prevalence of Salmonellosis. In other words, the age of the chickens has a contribution on their likelihood of being infected with salmonellosis (Table 3).
Table 3. Prevalence of Salmonellosis in the age category of poultry
|
No |
Age |
No of tested |
No.of positive |
Prevalence |
X2 |
P-value |
|
1 |
Grower |
140 |
55 |
39.3 |
|
|
|
2 |
Pullet |
138 |
50 |
36.2 |
6.9 |
0.03 |
|
3 |
Layer |
106 |
13 |
12.3 |
|
|
|
4 |
Total |
384 |
118 |
30.7 |
|
|
Table 4. Prevalence of Salmonellosis in Poultry housing system
|
No |
Housing system |
No of tested |
No.of positive |
Prevalence |
X2 |
P-value: |
|
1 |
Cage system |
100 |
25 |
25 |
|
|
|
2 |
Litter system |
284 |
93 |
32.7 |
1.4 |
0.35 |
|
3 |
Total |
384 |
118 |
30.7 |
|
|
Table 5. Prevalence of Salmonellosis in feed type and availability for Poultry
|
No |
Feed type |
No of tested |
No.of positive |
Prevalence |
X2 |
P-value: |
|
1 |
Commercial |
170 |
70 |
41.2 |
|
|
|
2 |
Locally |
214 |
48 |
22.4 |
11.0 |
0.000 |
|
3 |
Total |
384 |
118 |
30.7 |
|
|
Table 6. Prevalence of Salmonellosis in Poultry farm size
|
No |
Farm size |
No of tested |
No.of positive |
Prevalence |
X2 |
P-value |
|
1 |
Small |
142 |
9 |
6.3 |
|
|
|
2 |
Medium |
142 |
44 |
30.9 |
10.4 |
0.005 |
|
3 |
large |
100 |
65 |
65 |
|
|
|
4 |
Total |
384 |
118 |
|
|
|
Salmonellosis is one of the diseases affecting chicken breeding farms [13]. The identification of these diseases in Ethiopia raises significant concerns due to their potential for horizontal and vertical transmission [4]. In the current study, the overall prevalence of salmonellosis based on seropervalence was 30.7%. This finding was close agreement within finding with the works of other, 28.6% at Gondar [20]; Khudor et al., [21] reported 25.7% prevalence of Salmonella in breeding farms in Iraq.
In contrast, the present prevalence was lower 36.1% in broiler farms in Japan [22], and [23] in Nigeria, who reported a 43% prevalence rate among village chickens. This could be due to the smaller sample size used in the present study. This disagreement could be due to differences in environmental contamination, management systems used, smaller sample size, breed and parent stock difference of chickens used in the present, and those done by other researchers [24].
However, the finding was higher than those reported in other studies from Ethiopia: 15.12% in poultry farms from Modjo [25], 5.38% from from in and around Arba Minch town, Gamo Zone, Ethiopia [26], 1.5% prevalence at East Showa, Ethiopia of [24], and [22] who reported 10.05% seroprevalence in layers and pullets in Debre-Zeit poultry farms respectively. It is important to note that these studies were conducted in different regions of Ethiopia and used different sampling methods, so the prevalence rates may vary depending on the location and methodology used [14].
The current finding revealed that, three of the risk factors including the age, source of feed and farms size were found to be statistically significant (p < 0.05) factors for Salmonella prevalence. The prevalence of salmonellosis in Ethiopian poultry shows variations across different studies, with factors such as age, housing system, feed type, and farm size contributing to discrepancies in its occurrence [26]. This showed that the necessity for more comprehensive and specific research to understand the impact of these factors and develop effective control and prevention strategies.
The current finding showed that when considering the feed type, chickens fed with commercial feed had a higher proportion of positive cases compared to those fed with locally formed feed. This finding was agreement within previous finding of [26] from in and around Arba Minch town, Gamo Zone, Ethiopia. Salmonella infection in breeding farms may result from household waste products that may have come into contact with other Salmonella sources as a result of contact with a different production system [22].
The current studies showed that, there was a significant association between farm size and the occurrence of salmonellosis, Salmonellosis was most commonly prevalent in farm with large flock, at 65%, followed by medium farm 30.9% and small scale (6.3%). This finding was supported by reported of [26] from from in and around Arba Minch town, Gamo Zone, Ethiopia, [27] London, United Kingdom, [28] of central and south Ethiopia, [29] in Debre-Zeit, [30] in Debre Zeit and Modjo, Ethiopia, [31] in Shoal and Denbi State poultry farms. The age ability of infection to survive increases with age and mortality is greatest in newly hatched chicks [26].
CONCLUSION AND RECOMMENDATION
The present study recorded an overall prevalence of 30.7%, which might entail that salmonellosis was a major health problem of poultry farm which undoubtedly will have a drawback on the productivity. The current finding revealed that, three of the risk factors including the age, source of feed and farms size were found to be statistically significant (p < 0.05) factors for Salmonella prevalence. In conclusion the salmonellosis in study area was moderate. However, in developing countries like Ethiopia the prevalence of salmonellosis remains a challenge.
Based on the above conclusion, the following recommendations were forwarded:
- Should be implement comprehensive disease surveillance and monitoring program to track the prevalence of salmonellosis in poultry farms in Wolaita Sodo town.
- Should be regular testing and monitoring of poultry flocks can help to identify infected birds and take appropriate measures to prevent the spread of the disease.
- Should be promote good management practices in poultry farms, including proper sanitation, biosecurity measures, and vaccination programs. This can help to reduce the risk of Salmonella infection and improve overall flock health.
- Should be conduct awareness campaigns to educate poultry farmers and the general public about the importance of hygiene, safe handling of poultry products, and the risks associated with Salmonella infection. This can help to minimize the transmission of Salmonella from poultry to humans.
- Should be invest in research and development activities to understand the factors contributing to the prevalence of salmonellosis in poultry farms in the study area. This can include studying the impact of feed types, farm sizes, and management systems on disease prevalence and identifying effective control measures.
- Should be foster collaboration between veterinary authorities, poultry farmers, and relevant stakeholders to address the challenges of salmonellosis in poultry. This can involve capacity-building programs, knowledge sharing, and support for implementing best practices in disease control.
DECLARATION
Ethics approval and Consent to participate
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Availability of data and materials
All the datasets generated or analyzed during this study are included in this manuscript.
Competing interests
All authors have nothing to disclose in this work.
Funding
The current study was not funded by any institution.
AUTHORS' CONTRIBUTIONS
All authors contributed to data collection, study design, data interpretation, reference search, manuscript writing, and editing, and all authors have approved the submission of the final manuscript.
REFERENCES
- Guèye EF. (2005). Gender aspects in family poultry management systems in developing countries. World's Poultry Science Journal. 61(1):39-46.
- Moreki JC, Dikeme R, Poroga B. (2010). The role of village poultry in food security and HIV/AIDS mitigation in Chobe District of Botswana. Livestock Research for Rural Development. 22(3):1-7.
- Central Agricultural Census Commission (CACC). (2003). Statistical report on farm management practices, livestock, and farm implements. Part II. The Federal Democratic Republic of Ethiopia.
- Bouzoubaa K, Lemainguer K, Bell JG. (1992). Village chickens as a reservoir of Salmonella Pullorum and Salmonella Gallinarum in Morocco. Preventive Veterinary Medicine. 12:95-100.
- World Bank. (2008). The World Bank Group, World Development Indicators.
- Food and Agriculture Organization (FAO). (2007). Poultry sector country review-Ethiopia. FAO Animal Production and Health.
- Tadele G, Asrade B, Bayleyegn G, Sanni AM. (2014). Sero-prevalence of Fowl Typhoid and Pullorum Disease from Apparently Healthy Chickens in Ethiopia. J Veterinar Sci Technolo. 5(1):1000156.
- Kumari D, Mishra SK, Lather D. (2013). Pathomicrobial studies on Salmonella Gallinarum infection in broiler chickens. Veterinary World. 6(10):725-729.
- World Health Organization (WHO). (2010). Isolation of Salmonella species from food and animal feces. pp. 1-18.
- Abayneh E, Goba H, Shurbe M. (2023). Salmonellosis prevalence and risk factors in chicken breeding farms in and around Arba Minch town, Gamo Zone, Ethiopia. J Infect Dev Ctries. 17:226-235.
- Popoff MY, LeMinor L. (2001). Antigenic formulas of the Salmonella serovars (8th ed.). Paris, France: World Health Organization Collaborating Centre for Reference and Research on Salmonella.
- Antunes P, Mourão J, Campos J, Peixe L. (2016). Salmonellosis: the role of poultry meat. Clinical Microbiology and Infection. 22:110-121.
- Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Gast R, Humphrey TJ, et al. (2009). Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol Rev. 33(4):718-738.
- Rostagno MH, Wesley IV, Trampel DW, Hurd HS. (2006). Salmonella prevalence in market-age turkeys on-farm and at slaughter. Poult Sci. 85(10):1838-1842.
- Tadesse D, Singh H, Esatu AMW, Dessie T. (2013). Study on productive performances and egg quality traits of exotic chickens under the village production system in East Shewa, Ethiopia. African Journal of Agricultural Research. 8(13):1123-1128.
- Ahmad F, Ahsan-ul-haq AM, Hussain J, Siddiqui MZ. (2010). Production performance of White Leghorn hens under different lighting regimes. Pakistan Veterinary Journal. 30(1):21-24.
- Shivaprasad HL. (1997). Pullorum disease and fowl typhoid. In: Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, Eds. Diseases of Poultry. 10th Edition. Ames, IA: Iowa State University. pp. 82-96.
- Wray C, Davies RH. (2003). The epidemiology and ecology of Salmonella in meat-producing animals. In: Torrence ME, Isaacson RE, Eds. Microbial Food Safety in Animal Agriculture: Current Topics. 1st edn. USA: Blackwell Publishing. pp. 73-82.
- Thrust field M. (2018). Veterinary Epidemiology. 2nd edn. UK: Black well science Ltd. Pp. 182-118.
- Molla B, Alemayehu D, Salah W, Abinet T. (2019). Prevalence of salmonellosis in chickens in Gondar, Ethiopia. Journal of Veterinary Medicine. 10(3):123-135.
- Khudor MH, Ali AA, Nael MO. (2014). Isolation and identification of Salmonella spp. from poultry farms by using different techniques and evaluation of their antimicrobial susceptibilities. Bas J Vet Res. 1:246-259.
- Ishihara K, Takahashi T, Morioka A, Kojima A, Kijima M, Asai T, et al. (2009). National surveillance of Salmonella enterica in food-producing animals in Japan. Acta Vet Scand. 51(1):35.
- Adesiyun AA, Bishu G, Adegboye DS. (1984). Serological Survey of S. Pullorum Antibody in Chickens around Zaria, Nigeria. Bulletin of Animal Health and Production in Africa. 32:81-85.
- Smith RA, Mann R, McKinney AR. (2020). Seroprevalence of Salmonella in intensive poultry farming: A systematic review and meta-analysis. Poultry Science. 99(8):3819-3830.
- Abunna F, Bedasa M, Beyene T, Ayana D, Mamo B, Duguma R. (2017). Salmonella: isolation and antimicrobial susceptibility tests on isolates collected from poultry farms in and around Modjo, Central Oromia, and Ethiopia. JAPSC. 5:21-35.
- Aribam SD, Elsheimer-Matulova M, Matsui H, Hirota J, Shiraiwa K, Ogawa Y, et al. (2015). Variation in antigen-antibody affinity among serotypes of Salmonella O4 serogroup, determined using specific antisera. FEMS Microbiol Lett. 362(21):fnv168.
- Dereje N. (2002). Investigation on the identification of major disease of exotic chicken in three selected commercial poultry farms in Debre-Zeit. DVM thesis, Faculty of Veterinary Medicine, Addis Ababa University, Ethiopia.
- Mares M. (2017). Current topics in Salmonella and Salmonellosis. London, United Kingdom, IntechOpen. Available at: https://www.intechopen.com/books/5464.
- Fiseha M. (2015). Salmonella isolates and drug resistance epidemiology in poultry related samples in selected sites of central and south Ethiopia. MSc Thesis. Addis Ababa University. pp. 1-100.
- Destaw AA, Belege T, Aragaw E. (2020). Prevalence and antibiotic resistance pattern of Salmonella isolated from caecal contents of exotic chicken in Debre Zeit and Modjo, Ethiopia. Int J Microbiol. 6:1910630.
- Melese G. (1991). Epidemiological study of Salmonella pullorum and/or gallinarum infection in Shoal and Denbi State poultry farms. DVM thesis, Faculty of Veterinary Medicine, Addis Ababa University, Ethiopia.
.png)