Previous Issues Volume 9, Issue 1 - 2024
Radiotherapy Associated Oesophageal Vascular Ectasia with Acute Bleeding: A Case Report
Jan Kubovy*
Department of Gastroenterology and Hepatology, Christchurch Hospital, 2 Riccarton Avenue Christchurch, 4710, New Zealand
*Corresponding Author: Jan Kubovy, Department of Gastroenterology and Hepatology, Christchurch Hospital, 2 Riccarton Avenue Christchurch, 4710, New Zealand; Email: [email protected]
Received Date: June 19, 2024
Published Date: July 15, 2024
Citation: Kubovy J. © (2024). Radiotherapy Associated Oesophageal Vascular Ectasia with Acute Bleeding: A Case Report. Mathews J Gastroenterol Hepatol. 9(1):24.
Copyrights: Kubovy J. © (2024).
ABSTRACT
Gastrointestinal angiodysplasiae, also known as vascular ectasiae (VE) are not uncommon finding during endoscopy for any indication. Although frequently incidental, these lesions can cause iron deficiency anaemia, occult, or even overt gastrointestinal bleeding. The aetiology isn’t well understood with radiotherapy being one of only few known causalities of acquired VE. We present a unique case of radiotherapy related bleeding oesophageal VE. This is a first such case in literature. Its successful management utilized well established endoscopic thermal ablation method. Potential applications of other treatment modalities in such situation are also discussed.
Keywords: Angiodysplasia, Oesophagus, Bleeding, Radiotherapy
INTRODUCTION
Angiodysplasiae or vascular ectasiae (VE) are form of aberrant blood vessels. Gastrointestinal tract is relatively common site, although precise prevalence is unknown [1,2]. These lesions are often asymptomatic and are found incidentally during endoscopy for unrelated indications. However, VE may cause iron deficiency, occult, or even overt gastrointestinal bleeding [3]. VE can be congenital (or related to an inherited condition with delayed onset), however, vast majority are acquired. Several pathophysiological mechanisms have been proposed, but the aetiology of most remains uncertain. Radiotherapy is one of only few known causalities of acquired VE. Whilst oesophageal VE can be considered uncommon, bleeding oesophageal VE is certainly a rare finding with only very few published reports [4,5]. We present such case related to previous radiotherapy, causing overt upper gastrointestinal bleeding. The management of these lesions almost always involves endoscopic ablation. Our successful treatment of choice was one of commonly used thermal ablation techniques. In this paper we also contemplate application of other possible treatment modalities.
CASE REPORT
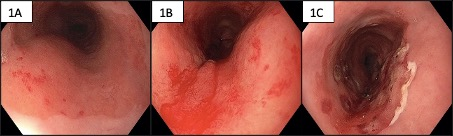
We present a 64-year-old Caucasian male with significant smoking history and a recent diagnosis of small cell carcinoma of distal oesophagus (limited stage), arising within a long segment Barrett’s oesophagus (Prague C8-M8). This rare type of cancer is considered an extrapulmonary equivalent of small cell lung cancer. No mucosal vascular abnormalities were noted during initial gastroscopy that had led to his cancer diagnosis. He has undergone a curative intent chemoradiotherapy with 4 cycles of carboplatin-etoposide and a total radiation dose of 45 Gy over 30 fractions. Restaging gastroscopy 8 months after completion of his oncological treatment revealed small hiatus hernia, 8 cm long Barrett’s oesophagus with scarring and few small VE at the proximal extent of Barrett’s mucosa (Figure 1A). This elective follow-up gastroscopy preceded his acute presentation below by 2 months.
Figure 1A-C: 1A upper extent of Barrett’s mucosa with VE; 1B bleeding VE within Barrett’s mucosa; 1C post APC ablation
At the time of his subsequent acute hospital admission, he complained of melaena for several days and was found to have severe anaemia accompanied by breathlessness and fatigue. He was hemodynamically stable despite a haemoglobin level of only 36 g/L. The mean corpuscular volume or red blood cells (MCV) was normal at 97 fL/L, but a blood film review suggested component of iron deficiency.
He was transfused five units of red blood cells and proceeded to urgent gastroscopy. This revealed fresh blood in distal oesophagus and gastric body due to active slow ooze from proximal end of Barrett’s mucosa (Figure 1B). The culprit was found to be several small VE corresponding in site and appearance to the lesions seen on recent elective gastroscopy. This was treated with Argon Plasma Coagulation (APC) probe (Straight Fire FiAPC probe 3000A, by ERBE) with a setting of 1 L/min and 40 Watts (Figure 1C). A re-staging CT scan at the time revealed no evidence of cancer recurrence but reported on a diffuse oesophageal thickening attributed to radiation related oesophagitis. The VE were eventually eradicated with APC over a course of 3 endoscopic sessions in monthly intervals. His haemoglobin has normalized following several iron infusions.
DISCUSSION
Management of bleeding oesophageal VE is seldom reported [4,5]. The obvious questions this case raises are the pathogenesis of these lesions and the appropriate management.
In our case, we postulate these lesions have arisen as a delayed consequence of radiotherapy within an area of previous radiation beam. We consider this a clinical parallel with the so called “radiation proctitis”, a radiation associated VE related to previous pelvic radiotherapy [6-8].
Interestingly, we found no VE within the squamous epithelium immediately above the upper extent of Barrett’s mucosa. Whether this was simply due to the radiation field encompassing by chance only the Barrett’s mucosa or whether oesophageal squamous epithelium is inherently more resistant to radiation induced neo-angiogenesis causing radiation associated VE is unclear. Anecdotally, we don’t often see radiation associated VE within the oesophageal squamous mucosa following radiotherapy for oesophageal squamous cell carcinoma despite this being a commonly applied treatment modality. Our literature search certainly did not find any such published cases. We therefore wonder whether radiation associated neo-angiogenesis could have a different course in columnar mucosa such as the one found in rectum or Barrett’s oesophagus versus in squamous epithelium. We found no published reports to support or refute this theory.
In terms of our chosen treatment modality, APC is widely used, effective and safe method to treat VE of gastrointestinal tract in any location [9-11]. It’s application for this particular indication is obviously scarce. However, APC use within oesophagus is a standard modality for Barrett’s mucosa eradication, either in addition or instead of radiofrequency ablation (RFA) and endoscopic resection. Some advocate the use of “hybrid APC” in this setting. This method utilises submucosal lifting agent injection under the area of intended APC application, both to reduce risk of perforation and to allow for safe maximum tissue ablation. Hybrid APC isn’t as widely used outside Barrett’s or perhaps colonic VE ablation. We chose not to use hybrid APC partly because we suspected radiation induced submucosal fibrosis would preclude successful mucosal lift, but mostly due to the fact we had a good position with the APC probe tip parallel with the mucosa, thus reducing the potential perforation risk related to the powerful jet of argon gas.
A total of three treatment sessions were required to fully ablate the VE in our case. Repeat procedures are also often required in other sites such as gastric antral vascular ectasia (GAVE); Barrett’s oesophagus; so called “radiation proctitis” and sometimes even colonic VE.
Considering other treatment modalities in our acute bleeding case: Adrenalin injection alone might temporarily stop the bleeding, but a second modality would still be necessary to ablate the lesion to reduce subsequent risk of recurrent bleed. Same issue arises with topical haemostatic agents such as Hemospray (Endoscopic Hemostat by Cook Medical). A mechanical device such as standard through the scope clip would be unlikely to deal with anything beyond a single large bleeding vessel. Other forms of thermal ablation such as RFA could certainly be entertained, but this is expensive, cumbersome, and imprecise method for treating localized bleeding VE. A cryoablation is another emerging modality that might become pertinent in near future. Some GAVE cases are treated with mucosal banding, however, the suspected radiation associated submucosal fibrosis could increase the risk of perforation with this method in mid oesophagus. Lastly, we didn’t consider any pharmacological therapies such as thalidomide or bevacizumab. Their slow onset of action and, at best modest efficacy were deemed ill-suited in the case of acute bleeding.
REFERENCES
- Foutch PG, Rex DK, Lieberman DA. (1995). Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 90(4):564-567.
- Notsu T, Adachi K, Mishiro T, Kishi K, Ishimura N, Ishihara S. (2021). Prevalence of Angiodysplasia Detected in Upper Gastrointestinal Endoscopic Examinations. Cureus. 13(4):e14353.
- García-Compeán D, Del Cueto-Aguilera ÁN, Jiménez-Rodríguez AR, González-González JA, Maldonado-Garza HJ. (2019). Diagnostic and therapeutic challenges of gastrointestinal angiodysplasias: A critical review and viewpoints. World J Gastroenterol. 25(21):2549-2564.
- Crone V, Bremholm L. (2017). Oesophageal vascular ectasia in a patient with anaemia. Ugeskr Laeger. 179(21):V11160858.
- Khanna S, Arora AS, Topazian MD. (2011). Esophageal vascular ectasia. Endoscopy. 43(S 02):E281-.
- Feakins R. (2020). Radiation and the Gastrointestinal Tract. In: Feakins RM, editor. Non-Neoplastic Pathology of the Gastrointestinal Tract: A practical Guide to Biopsy Diagnosis. Cambridge: Cambridge University Press. 30-51.
- Takeuchi H, Kimura T, Okamoto K, Aoyagi E, Miyamoto H, Kaji M, et al (2012). A mechanism for abnormal angiogenesis in human radiation proctitis: analysis of expression profile for angiogenic factors. J Gastroenterol. 47:56-64.
- Ahmed M, Ahmed R. (2022). Radiation in gastroenterology. Gastroenterol Res. 15(6):285.
- Rosenfeld G, Enns R. (2009). Argon photocoagulation in the treatment of gastric antral vascular ectasia and radiation proctitis. Canadian J Gastroenterol Hepatol. 23:801-804.
- Peng M, Guo X, Yi F, Shao X, Wang L, Wu Y, et al. (2021). Endoscopic treatment for gastric antral vascular ectasia. Ther Adv Chronic Dis. 12:20406223211039696.
- Chiu YC, Lu LS, Wu KL, Tam W, Hu ML, Tai WC, et al. (2012). Comparison of argon plasma coagulation in management of upper gastrointestinal angiodysplasia and gastric antral vascular ectasia hemorrhage. BMC Gastroenterol. 12(1):1-7.