Information Links
Related Conferences
Previous Issues Volume 8, Issue 2 - 2024
Preparation and in Vitro Evaluation of Metronidazole Floating Microspheres
Ezegbe Chekwube Andrew1,4,*, Anikwe Celestine Chidera2, Adaka Uchechukwu Bill1, Ezegbe Amarachi Grace3,#, Okafor Nnedimma Pauline1, Odo Kenechi Benjamin1, Anyoha Cross-Raphael Chukwuebuka1, Ugorji Anita Chidera1, Okorafor Ezinne Chinemerem5, Onunkwo Chukwunwike Godswill1
1Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nsukka, Enugu, Nigeria
2Department of Clinical, Pharmaceutical and Biological Sciences, University of Hertfordshire, England, United Kingdom
3Department of Home Science and Management, University of Nigeria, Nsukka, Enugu State.
4Human and Natural Science Center, ABC Federal University, Santo Andre, Sao Paulo, Brazil
5Department of Pharmacology, Federal University of Technology, Owerri, Nigeria
*Corresponding author: Ezegbe Chekwube Andrew, Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nsukka, Enugu, Nigeria & Federal University of ABC (UFABC), Center for Natural and Human Sciences, Santo Andre, Brazil, Tel: +2348038042802, Email: [email protected]
#Co-corresponding author: Ezegbe Amarachi Grace, Department of Home Science and Management, University of Nigeria, Nsukka, Enugu State, Nigeria, Tel: +2348061114433, E-mail: [email protected]
Received Date: July 04, 2024
Published Date: September 20, 2024
Citation: Andrew EC, et al. (2024). Preparation and in Vitro Evaluation of Metronidazole Floating Microspheres. Mathews J Pharma Sci. 8(2):32.
Copyrights: Andrew EC, et al. © (2024).
ABSTRACT
Introduction: Over the years, metronidazole floating microspheres have been used to prolong the gastric residence time after oral administration and in the treatment of certain diseases such as peptic ulcer and other gastrointestinal infections. Aim: Development and in vitro evaluation of metronidazole floating microspheres in the treatment of gastrointestinal infections. Methods: The microspheres were formulated using inotropic gelation method. A homogenous polymer solution was prepared by dissolving sodium alginate (1 g) and the polymers (1 g each) in 32 ml of distilled water. The drug was added to the polymer solution and stirred continuously to form a viscous dispersion. A 10 % w/v quantity of Cacl2 solution was prepared and used as a cross–linking agent. The dispersion was added drop wise for 15 minutes for the curing reaction to take place. The spheres obtained were then washed and dried at 45 oC for 12 hours. Results: The percentage yield of the formulations ranged from 72.50 % ± 1.77 to 84.50 % ± 3.18. The angle of repose ranged from 3.25o ± 0.04 to 6.85o ± 0.32, while the bulk density ranged from 0.40 g/ml ± 0.07 to 0.64 g/ml ± 0.01. The Carr’s index ranged from 13.04 % ± 0.04 to 15.04 % ± 0.01. The swelling index ranged from 62.00 % ± 0.71 to 83.50 % ± 0.35 without a significant difference (p < 0.05). The in vitro drug release showed that formulation F-5 gave the least release at 28.75 % ± 0.81 after 4 hours. Conclusion: The formulation F-5 was chosen as the most appropriate formulation from all tested and was optimized.
Keywords: Metronidazole, Microspheres, Gastrointestinal Infection, Gastric Residence Time.
INTRODUCTION
Floating drug delivery systems are classified as dosage forms that have the capacity to remain afloat in the stomach region over an extended period of time [1]. Some of the unique properties associated with floating drug delivery system include increased gastric residence time, enhanced drug solubility and bioavailability [2]. Gastric retention is usually affected by the specific gravity of food, frequency of food intake and caloric content [3]. For the floating system to remain buoyant without affecting the gastric emptying rate, there must be high level of fluids in the stomach. Bioactive polymers are used in coating floating dosage forms [4]. This coating ensures that they adhere to the mucous lining of the walls in the stomach and also facilitates the efficiency of drug delivery. Floating dosage forms have been evaluated in many dosage forms like tablets, capsules, microparticles, granules and beads. In the development of controlled drug delivery system, the major challenge is usually on how to prolong the drug release over a period of time. Among all the different routes of drug delivery, the oral route is the most common [5]. Gastroretentive floating microspheres are known low density systems that have the ability to maintain buoyancy to float over a prolonged period of time [6]. There are various types of microspheres that are used for drug delivery. They include the bioadhesive microspheres, magnetic microspheres, polymer microspheres, radioactive and floating microspheres [7].
This study was based on the preparation and in vitro evaluation of metronidazole floating microspheres using different polymers in order to achieve controlled drug delivery and a focus on optimizing drug release kinetics and enhancing therapeutic efficacy. The controlled drug delivery will enhance patients’ compliance to their medication, reduce side effects and improve the overall well-being of patients.
MATERIALS AND METHODS
Metronidazole (MET/4/2008/0076C), was purchased from Emzor Pharmaceuticals Ltd, Lagos. Sodium alginate, ethyl cellulose, sodium carboxy methyl cellulose (Na-CMC), were purchased from (Sigma Aldrich, Kosher, USA). Methanol was obtained from (Astron Chemicals, Ahmedabad). Glycerin and sodium hydroxide were provided by (Mingtai Chemical Taiwan). Calcium chloride was obtained from (Evonik, Germany). Hydroxy propyl methyl cellulose (HPMC) were obtained from (DFE Pharma, UK), Sorbitol was obtained from (TCI, USA). Distilled water was obtained from (UNN Water Resources Management Laboratories Ltd; UNN, Enugu State, Nigeria). All chemicals used were of analytical grade.
Method of preparation
Orifice inotropic gelation method was used for the preparation of metronidazole microspheres using polymers such as ethyl cellulose (EC), sodium carboxyl methyl cellulose (Na-CMC), hydroxyl propyl methyl cellulose (HPMC) and sodium alginate [8]. A homogenous polymer solution was prepared by dissolving sodium alginate (1g) and the polymers (1g) in purified water (32 ml). Metronidazole (1g), the active substance was added to the polymer solution and stirred thoroughly to form a viscous dispersion. A 10 % w/v quantity of calcium chloride solution was prepared which was used as a cross linking agent. The prepared dispersion was then manually added drop wise into calcium chloride (10 % w/v) solution (40 ml) using a syringe having a needle of size no.18. The calcium chloride solution having the droplets was then allowed to stay for 15 minutes for the curing reaction to take place and produce spherical rigid drug loaded spheres. The spheres obtained after the reaction were then collected and washed repeatedly with acetone. After washing, the spheres were properly dried at 45oC for 12 hours.
Table 1. Composition of formulations
|
Ingredients (g)/Batches |
F-1 |
F-2 |
F-3 |
F-4 |
F-5 |
F-6 |
F-7 |
F-8 |
F-9 |
|
Metronidazole |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Sodium alginate |
1 |
1 |
0.8 |
1 |
0.8 |
1 |
0.8 |
1 |
0.8 |
|
Ethyl cellulose |
1 |
0.2 |
1 |
- |
- |
- |
- |
- |
- |
|
Na-CMC |
- |
- |
- |
1 |
0.2 |
1 |
- |
- |
- |
|
HPMC |
- |
- |
- |
- |
- |
- |
1 |
0.2 |
1 |
|
Acetone (ml) |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
|
Cacl2 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
The relative yield was calculated based on the amount of microspheres of each formulation obtained relative to the amount of solid materials used in the dispersed phase [9]. The percentage yield was calculated according to the following equation [10]:
Pre-compression evaluation of powder blend
Angle of repose
Angle of repose is defined as the maximum angle possible between the surface of a pile of the powder and the horizontal plane. It involves the use of a plastic funnel in ring-supported by a retort stand. A sheet of paper was placed below the funnel assembly. A sheet of fibre board was placed below the funnel orifice making sure it fits tightly. A given quantity of the microsphere (30 g) was transferred into the funnel. The fibre sheet was drawn away and the timer simultaneously started. The timer was stopped when all of the powder had passed through the funnel. The height of the heap was measured using a graduated ruler. A pencil was used to outline the base of the contour. The angle of the conical heap so formed was determined from equation 5. The powder was returned to the funnel and the experiment was repeated thrice [10].
Bulk density
This is the ratio between a given mass of powder and its bulk volume. A weighed quantity of the microsphere (30.0 g) was placed in a 100-ml graduated cylinder. The cylinder was gently dropped onto a wooden surface three times from a height of one inch at 2 sec intervals. The volume assumed after the treatment was taken as the bulk volume. The experiment was repeated [10]:
Tapped density
This is the ratio between a given mass of powder and its bulk volume. A weighed quantity (30.0 g) of the powder was placed in a 100-ml graduated cylinder. The cylinder was tapped up to 500 times on the wooden surface or to a constant volume. The final volume attained represents the tapped volume. The experiment was repeated thrice [10]
Carr’s index
This is used to access the flowability of a powder. The Carr’s compressibility index (CI %) was calculated from the poured (bulk density) and tapped densities. CI was calculated using the following equation:
Hausner’s ratio
The Hausner’s ratio (HR) is the ratio of tapped to bulk densities. It is a common technique widely used to describe the packing behavior of powders when they are subjected to tapping [10]
Swelling index
The weight of the microspheres was taken and then dispersed in phosphate buffer (pH 7.2) for 12 hours. The excess liquid was removed using blotting paper and the weight of the swollen microspheres taken. The swelling index was calculated thus [11]:
Drug content
A 1g quantity of sample was taken and dissolved in 100 ml distilled water in a beaker. After 24 hours, the sample was filtered and suitable dilution was done. Then the absorbance of the solution was measured at 277 nm and drug content was calculated [12].
In vitro analysis
A 500 ml quantity of distilled water was placed in the dissolution apparatus (USP apparatus type-II paddle method). The sample was then placed in the vessel and the apparatus was operated for 4 hours at 50 resolutions per minute (RPM). At a definite time interval, 5 ml was withdrawn from the vessel and another 5 ml of the blank was added to the vessel. The withdrawn fluid was then filtered and suitable dilution was done. Samples were analyzed under UV Spectrophotometer at 277 nm (V-1200, DFE Pharma, UK) [13].
Morphology of the metronidazole microspheres
The morphology of the obtained microspheres was examined under a light microscope (Zeiss, Me 63 C, West Germany) with varied magnification powers. One drop of the freshly prepared microsphere suspension was poured onto a slide and sealed with a cover glass. Photomicrographs were captured using Samsung digital camera [14]. The morphology, size, uniformity and aggregation or coalescence of the microspheres were studied [14].
Drug-excipient compatibility study (FTIR spectroscopy)
Infra-red spectra of pure drug, carrier and coating materials were obtained by (Shimadzu 8400S Japan) FT-IR spectrometer. The samples were previously ground and mixed thoroughly with potassium bromide, an infra-red transparent matrix at 1:5 (sample: KRr) ratio respectively. The KBr discs were prepared by compressing the powders at a pressure of 5 tons for 5 minutes in a hydraulic press. The scans were obtained at a resolution of 4 cm-1 from 4000 to 400 cm-1 [15].
Data analysis
All the measurements were repeated at least thrice and the data obtained analyzed by Student t-test and One-Way Analysis of Variance (ANOVA). Statistical analysis was performed using Statistical Product and Services Solution software (SPSS, version 22.0 Inc., Chicago IL, USA) and Excel Microsoft Office version 2012. The results were presented as mean ± SD, and statistical differences between means considered significant at (p < 0.05)
RESULTS AND DISCUSSION
Percentage yield of the metronidazole microspheres
The percentage yield of the metronidazole microspheres varied from 72.50 % ± 1.77 to 84.50 % ± 3.18 without any significant difference (p < 0.05) at different concentrations of the drug-polymer ratios. Batches F-5 and F-7 recorded the lowest percentage yield at 72.50 % ± 1.77 and 72.50 % ± 1.77 respectively without any significant difference (p < 0.05). According to Trivedi et al, the reduction in the percentage yield with increasing drug-polymer ratio may be due to the loss of smallest particles during filtration and washing [15].
Table 2. Percentage yield of metronidazole microspheres
|
Formulation code |
Yield (%) ± SD |
|
F-1 |
81.00 ± 2.05 |
|
F-2 |
82.50 ± 1.77 |
|
F-3 |
74.50 ± 1.06 |
|
F-4 |
80.50 ± 4.60 |
|
F-5 |
72.50 ± 1.77 |
|
F-6 |
83.00 ± 2.12 |
|
F-7 |
72.50 ± 1.77 |
|
F-8 |
84.50 ± 0.35 |
|
F-9 |
84.50 ± 3.18 |
The angle of repose is an indicator of the internal friction or cohesion between particles. A high angle of repose suggests a cohesive powder, while a low value indicates a non-cohesive powder [17]. The results showed the angle of repose for the metronidazole floating microsphere formulations ranged from 4.30° ± 0.11 for batch F-4 to 6.63° ± 0.16 for batch F-7 without a significant difference (p < 0.05). These low values indicated good flow and cohesive properties of the powders [17].
Bulk density
Bulk density provides a measure of the flow properties of a powder, which is influenced by particle size and distribution [17]. A higher bulk density correlates with better flow characteristics. The bulk density values obtained were in the range of 0.40 g/cm3 ± 0.07 for batch F-3 to 0.64 g/cm3 ±0.01 for batch F-8 without a significant difference (p < 0.05).
Tapped density
Tapped density is dependent on particle size and size distribution. The tapped density values ranged from 0.46 g/cm3 ± 0.03 for batch F-3 to 0.67 g/cm3 ± 0.02 for batch F-7 without a significant difference (p < 0.05).
Carr's index
The Carr's index (CI) indicates the flow properties of a powder. Values between 5-15 % represents excellent flow and 12-16 % represents good flow according to BP specifications [17]. Powders with CI above 38 % are considered very poor flowing and cohesive. The CI values for the microsphere formulations were between 13.04 % ± 0.04 (batch F-6) and 15.04 % ± 0.01 (batch F-4), without a significant difference (p < 0.05) suggesting good to excellent flow properties.
Hausner's ratio
The Hausner's ratio (HR) provides another measure of powder flow, with a ratio between 1.00-1.11, indicating excellent flow and 1.12-1.18, representing good flow properties. HR values above 1.6 are characteristic of very poor, cohesive flow powders/granules. The HR ranged from 1.04 ± 0.00 for batch F-4, showing excellent flow, to 1.29 ± 0.08 for batch F-8, without a significant difference (p < 0.05). The result obtained was within the range for good flow according to the British Pharmacopeia specifications [17].
Table 3. Summary of the angle of repose, bulk density, tapped density, Carr's index, and Hausner's ratio
|
Formulation code |
Angle of repose (o) |
Bulk density (g/ml) |
Tapped density (g/ml) |
Carr’s Index (%) |
Hausners ratio |
|
F-1 |
6.85 ± 0.32 |
0.41 ± 0.07 |
0.46 ± 0.03 |
14.43 ± 0.26 |
1.04 ± 0.00 |
|
F-2 |
3.45 ± 0.04 |
0.40 ± 0.07 |
0.62 ± 0.01 |
14.00 ± 0.64 |
1.05 ± 0.00 |
|
F-3 |
9.20 ± 0.21 |
0.40 ± 0.07 |
0.59 ± 0.01 |
14.33 ± 0.26 |
1.04 ± 0.01 |
|
F-4 |
4.50 ± 0.00 |
0.41 ± 0.00 |
0.52 ± 0.01 |
15.04 ± 0.01 |
1.05 ± 0.00 |
|
F-5 |
3.25 ± 0.04 |
0.52 ± 0.01 |
0.67 ± 0.01 |
14.95 ± 0.25 |
1.08 ± 0.02 |
|
F-6 |
4.35 ± 0.11 |
0.64 ± 0.01 |
0.64 ± 0.01 |
13.04 ± 0.04 |
1.14 ± 0.01 |
|
F-7 |
5.08 ± 0.01 |
0.57 ± 0.02 |
0.66 ± 0.02 |
13.49 ± 0.29 |
1.15 ± 0.01 |
|
F-8 |
6.63 ± 0.16 |
0.55 ± 0.02 |
0.63 ± 0.02 |
13.09 ± 0.29 |
1.29 ± 0.08 |
|
F-9 |
6.58 ± 0.19 |
0.64 ± 0.00 |
0.61 ± 0.02 |
13.17 ± 0.62 |
1.36 ± 0.01 |
The metronidazole microspheres floated for prolonged period of time when it was immersed on the dissolution medium. The percentage of the swelling index was highest for formulations with HPMC and sodium alginate polymers. There could be a direct relationship between the increase in polymer concentration and increased buoyancy time [18]. According to Chintapalli et al, an increase in polymer concentration led to an increase in the buoyancy time [18]. The swelling index was used to determine the amount of phosphate buffer absorbed by the microspheres after dissolving them in the buffer. From the results obtained, it ranged from 69.00 ± 0.00 to 83.50 ± 0.35 for batches F-2 and F-9 respectively without any significant difference (p <0.05). According to Oluwatoyin et al, the swelling index of the microspheres also increased with increase in the concentration of starch in the polymer blend. The metronidazole microspheres showed significant (p < 0.05) higher swelling index than those containing sodium alginate alone as the polymer [19]. The drug content was found to be between 18.50 ± 9.55 to 45.50 ± 1.77 for F-7 and F-9 respectively. This depicts that the formulation F9 containing HPMC polymer gave the highest drug content, while F-7 gave the least drug content of 18.50 % ± 9.55.
Table 5. Swelling index and Drug content of metronidazole microspheres (mean ± SD)
|
Formulation code |
Swelling index (%) |
Drug content (%) |
|
F-1 |
62.00 ± 0.71 |
26.33 ± 1.25 |
|
F-2 |
69.00 ± 0.00 |
28.33 ± 2.02 |
|
F-3 |
68.00 ± 2.83 |
26.50 ± 1.06 |
|
F-4 |
71.50 ± 1.06 |
29.00 ± 1.41 |
|
F-5 |
78.50 ± 1.77 |
29.00 ± 2.12 |
|
F-6 |
75.00 ± 3.54 |
31.50 ± 1.06 |
|
F-7 |
82.00 ± 1.41 |
18.50 ± 9.55 |
|
F-8 |
83.50 ± 0.35 |
31.50 ± 1.06 |
|
F-9 |
83.50 ± 0.35 |
45.50 ± 1.77 |
The drug release profile of the metronidazole floating microspheres are found in Table 6. The drug release profile of the floating microspheres increased with time. According to Huang et al, for controlled release preparations, an initial high rate of drug release is usually observed at the beginning of the controlled release process which could be due to a number of mechanisms such as surface desorption, pore diffusion and lack of a diffusion barrier to regulate the diffusion process [20]. The results obtained showed that the time taken for at least 20 % of the metronidazole release were higher than 60 minutes. This indicated that the metronidazole microspheres did not show any sign of burst release, thus indicating that they might had been embedded in the microspheres [21]. From the results obtained, formulation F-7 gave the highest cumulative drug release of 61.00 % ±0.71 at 240 minutes, while formulation F-5 containing HPMC provided the lowest release of 28.75 % ± 0.81 over the same period.
Table 6. Drug release profile of metronidazole microspheres (mean ± SD)
|
Time (mins)/Batches |
F-1 |
F-2 |
F-3 |
F-4 |
F-5 |
F-6 |
F-7 |
F-8 |
F-9 |
|
0 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
15 |
2.45 ± 0.32 |
2.00±0.28 |
2.60±0.07 |
2.30±0.08 |
1.90±0.21 |
2.05±0.04 |
2.65±0.11 |
2.75±0.53 |
2.20±0.49 |
|
30 |
7.95 ± 0.32 |
8.25±0.18 |
8.10±0.21 |
7.43±0.34 |
8.00±0.00 |
8.45±0.04 |
8.10±0.14 |
7.75±0.46 |
7.55±0.39 |
|
60 |
17.0 ± 1.41 |
12.45±0.32 |
18.00±2.12 |
16.20±1.30 |
12.45±0.11 |
7.10±3.32 |
16.50±1.06 |
16.65±1.17 |
15.35±0.46 |
|
90 |
34.0 ± 1.41 |
15.30±0.21 |
37.00±0.71 |
27.30±2.49 |
14.65±0.18 |
15.75±0.74 |
36.85±0.81 |
26.00±1.41 |
16.95±0.46 |
|
120 |
46.0 ± 0.71 |
18.07±0.07 |
43.00±1.41 |
35.83±2.25 |
18.05±0.04 |
18.30±0.21 |
36.50±0.35 |
35.50±0.35 |
19.65±0.25 |
|
150 |
52.0 ± 3.54 |
21.05±0.74 |
50.50±2.47 |
42.43±2.19 |
20.90±0.64 |
19.65±0.11 |
43.00±2.12 |
41.50±3.18 |
25.25±1.59 |
|
180 |
58.5 ± 6.01 |
23.70±0.49 |
52.00±0.00 |
49.93±2.37 |
13.10±6.79 |
23.65±0.46 |
50.50±0.35 |
47.50±1.77 |
33.00±2.12 |
|
210 |
62.0 ± 1.41 |
25.70±0.21 |
61.50±1.06 |
53.67±1.70 |
25.55±0.18 |
25.15±0.11 |
58.00±0.00 |
54.00±2.83 |
41.60±0.78 |
|
240 |
71.00±2.83 |
30.35±0.11
|
62.50±1.06 |
63.90±2.91 |
28.75±0.81 |
29.80±1.56 |
61.00±0.71 |
62.50±1.77 |
40.50±0.24
|
Figure 1. Cumulative percentage drug release of batches F-1 to F-3.
Figure 2. Cumulative percentage drug release of batches F-4 to F-6.
Figure 3. Cumulative percentage drug release of batches F-7 to F-9.
Figure 4. Cumulative percentage drug release of batches F-1 to F-6.
Scanning electron microscopy
The metronidazole microspheres were discrete and spherical in shape. Formulations F-1 and F-2 showed smoother surfaces than the microspheres that were formulated with HPMC (F-7 and F-9). According to Md et al, the presence of guar gum on the surface of the microspheres might cause a slightly rough surface as guar gum might interfere with the cross-linking of alginate by calcium ions [22].
Figure 5. SEM of batch F-1.
Figure 6. SEM of batch F-2.
Figure 7. SEM of batch F-7.
Figure 8. SEM of batch F-8.
FTIR compatibility studies
FTIR Spectroscopy (drug- excipient compatibility studies)
Figure 9 shows the characteristic peaks of metronidazole at 3831.0, 2523.5, 1823.0 and 1273.8 cm-1 corresponding to O-H single bond stretch, C-H single bond stretch, nitriles and carbenes, C=C, C=O and C-C, C-O single bond respectively.
Figure 10 shows the characteristics peaks of ethyl cellulose at 3925.2, 3245.2, 2582.1, 1997.8 and 1468.4 cm-1 corresponding to O-H, N-H single bond stretch, C-H single bond stretch, carbenes triple bond, C=O, C=C double bond and C-C, C-O single bond respectively. According to Sunil et al, the spectrum of EC showed characteristic peaks at 3390 and a band at 1636 cm-1 corresponding to the stretching and bending modes of the surface hydroxyls. The peak at 2905 cm-1 belongs to the asymmetrically stretching vibration of C-H in a pyramid ring and the broad absorption peak at 1059 cm-1 is attributed to the C-O of cellulose [23].
Figure 11 shows the characteristic peaks of sodium carboxy-methyl cellulose at 3852.3, 3169.1, 2554.2, 1993.8 and 1495.6 cm-1 corresponding to O-H, N-H single bond stretch, C-H single bond stretch, nitriles and carbenes triple bond, C=O, C=C double bond and C-O, C-C single bond respectively. According to Mastiholimath et al, the spectrum of Na-CMC showed characteristic peaks at 3700 cm-1 indicating the presence of –OH stretching bond. The strong bonds at 1093, 459 and 798 cm-1 were associated to the asymmetric and symmetric Si-O—Si stretching vibration bonding [24].
Figure 12 shows the characteristic peaks of HPMC at 3675.0, 3269.4, 2434.3, 1900.5 and 1428.8 corresponding to –O-H, single bond stretch, C-H single bond stretch, nitriles and carbenes triple bond, C=O C=C double bond, C-O, C-C single bond respectively. According to Shoufeng et al; the spectrum of starch showed characteristics peaks at 3448 for –OH stretching, 2930 for –CH stretching, 1646 for C-O bending associated with OH group, and 1381 cm-1 associated with –CH symmetric bending.
Figure 13 shows the characteristic peaks of calcium chloride at 3900.1, 3143.6, 2427.5, 1873.9 and 1454.0 corresponding to –OH, -NH single bond stretch, -CH single bond stretch, C=O, C=C and C-O, C-C single bond respectively. According to Yogesh et al, the twin peaks at 1577 and 1466 cm-1 were attributed to asymmetric carbohydrate (-COO) stretching vibration and symmetric carbohydrate vibration respectively, while peaks at 2917 and 2850 cm-1 were attributed to the –CH stretching vibration [26].
Figure 9. FTIR spectrum of ibuprofen.
Figure 10. FTIR spectrum of ethyl cellulose.
Figure 11. FTIR spectrum of Na-CMC.
Figure 12. FTIR spectrum of HPMC.
Figure 13. FTIR spectrum of Calcium chloride.
CONCLUSION
In this study, the metronidazole microspheres were successfully formulated using different synthetic polymers. Formulation F-5, was chosen as the most appropriate formulation from all tested. Further in vivo evaluation on the metronidazole microspheres is highly recommended.
ACKNOWLEDGEMENTS
The authors are thankful to the Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nsukka, Enugu State.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- Cohen-Wolkowiez M, Sampson M, Bloom BT, Arrieta A, Wynn JL, Martz K, et al. (2013). Determining population and developmental pharmacokinetics of metronidazole using plasma and dried blood spot samples from premature infants. Pediatr Infect Dis J. 32(9):956-961.
- de C Bergamaschi C, Berto LA, Venâncio PC, Cogo K, Franz-Montan M, Motta RH, et al. (2014). Concentrations of metronidazole in human plasma and saliva after tablet or gel administration. J Pharm Pharmacol. 66(1):40-47.
- Kusters JG, van Vliet AH, Kuipers EJ. (2006). Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 19(3):449-490.
- Romano M, Cuomo A. (2004). Eradication of Helicobacter pylori: a clinical update. MedGenMed. 6(1):19.
- Sahasathian T, Praphairaksit N, Muangsin N. (2010). Mucoadhesive and floating chitosan-coated alginate beads for the controlled gastric release of amoxicillin. Arch Pharm Res. 33(6):889-899.
- Chun MK, Sah H, Choi HK. (2005). Preparation of mucoadhesive microspheres containing antimicrobial agents for eradication of H. pylori. Int J Pharm. 297(1-2):172-179.
- Ishak RA, Awad GA, Mortada ND, Nour SA. (2007). Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-Helicobacter pylori therapy. J Control Release. 119(2):207-214.
- Chen YC, Ho HO, Liu DZ, Siow WS, Sheu MT. (2015). Swelling/floating capability and drug release characterizations of gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose. PLoS One. 10(1):e0116914.
- Asnaashari S, Khoei NS, Zarrintan MH, Adibkia K, Javadzadeh Y. (2011). Preparation and evaluation of novel metronidazole sustained release and floating matrix tablets. Pharm Dev Technol. 16(4):400-407.
- Bakowsky H, Richter T, Kneuer C, Hoekstra D, Rothe U, Bendas G, et al. (2008). Adhesion characteristics and stability assessment of lectin-modified liposomes for site-specific drug delivery. Biochim Biophys Acta. 1778(1):242-249.
- Bytzer P, O'Morain C. (2005). Treatment of Helicobacter pylori. Helicobacter. 10(Suppl 1):40-46.
- Shaikh R, Raj Singh TR, Garland MJ, Woolfson AD, Donnelly RF. (2011). Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 3(1):89-100.
- Borrelli F, Izzo AA. (2000). The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 14(8):581-591.
- Kesavan K, Nath G, Pandit JK. (2010). Sodium alginate based mucoadhesive system for gatifloxacin and its in vitro antibacterial activity. Sci Pharm. 78(4):941-957.
- Choi BY, Park HJ, Hwang SJ, Park JB. (2002). Preparation of alginate beads for floating drug delivery system: Effects of co(2) gas-forming agents. Int J Pharm. 239(1-2):81-91.
- Trivedi P, Verma AML, Garud N. (2008). Preparation and characterization of aceclofenac microspheres. Asian J Pharm. 2(2):110-115.
- British Pharmacopoeia. (2009). British Pharmacopoeia, vol. III. London. Her Majesty’s Stationery Office. pp. 6578-6585.
- Chintapalli GS, Snigdharani B, Sruti R, Somesu M, Kiran KB, Kirtimaya M. (2020). Design and evaluation of floating microspheres of ranitidine HCl. The Pharma Innovation Journal. 9(3):223-233.
- Oluwatoyin AO, Aderemi AA, Tolulope OA, Olufunke DA, Adenike O. (2017). Formulation of floating metronidazole microspheres using cassava starch (Manihot esculente) as polymer. Journal of Pharmaceutical investigation. 9(7):1-8.
- Huang X, Brazel CS. (2001). On the importance and mechanism of burst release in matrix-controlled delivery systems. J Control Rel. 73(2-3):121-136.
- Jha AK, Bhattacharya A. (2008). Preparation and in vitro evaluation of sweet potato blended sodium alginate microbeads. Adv Nat Appl Sci. 2(3):122-128.
- Lutful A, Tajnin A, Abdul M. (2016). Development of floating mucoadhesive microsphere for site specific release of metronidazole. Advanced Pharmaceutical Bulletin. 6(2):195-200.
- Sunil Jain K, Awasti, AM, Jain NK, Agarwal GP. (2007). Gastroretentive floating drug delivery. An overview. Drug Del Tech. 5(7):176-187.
- Mastiholimath VS, Dandagi PM, Gadad AP, Mathews R, Kulkarni AR. (2008). In vitro and in vivo evaluation of ranitidine hydrochloride ethyl cellulose floating microparticles. Journal of Microencapsulation. 25(5):307-314.
- Li S, Lin S, Chien YW, Daggy BP, Mirchandani HL. (2001). Statistical optimization of gastric floating system for oral controlled delivery of calcium. AAPS Pharm Sci Tech. 2(1):123-130.
- Gattani YS, Bagawath DA, Maske AP. (2008). Formulation and evaluation of intragastric floating drug delivery system of diltiazem hydrochloride. Asian Journal of Pharmaceutics. 3(4):228-231.
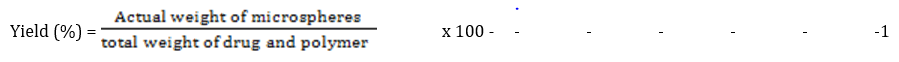
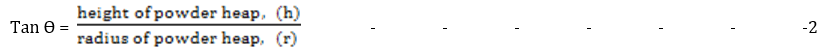


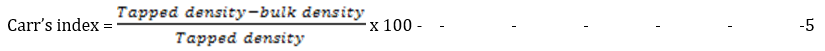
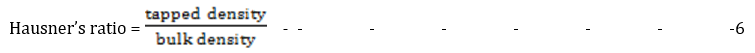
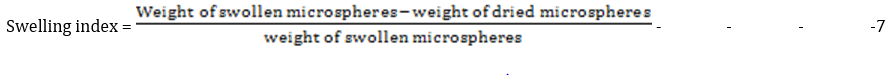
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)