Previous Issues Volume 4, Issue 1 - 2020
Prenatal Osteogenesis of the Long Bones of the Hindlimb in Sokoto Red Goat: Gross Osteomorphometry, Radiography and Histology
Danmaigoro A1*, Faruk AK1, Atabo SM1, Bello A1, Mahmud MA1,2, Abubakar K3, Mailafiya MM3
1Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto P.M. B 2346, Sokoto, Nigeria 2Department of Animal Health and Production Technology, Niger State College of Agriculture, P.M.B 109, Mokwa, Niger State, Nigeria 3Department of Human Anatomy, College of Medical Sciences, Federal University Lafia,950101, Akunza, Lafia, Nasarawa State, Nigeria
*Corresponding author: Abubakar K, Department of Human Anatomy, College of Medical Sciences, Federal University Lafia,950101, Akunza, Lafia, Nasarawa State, Nigeria, Tel: +234-803-791-4241; E-mail: [email protected]
Received Date: November 25, 2020 Published Date: December 27, 2020 Copyright: Danmaigoro A, et al. © 2020 Citation: Danmaigoro A, et al. (2020). Prenatal Osteogenesis of the Long Bones of the Hindlimb in Sokoto Red Goat: Gross Osteomorphometry, Radiography and Histology. Mathews J Vet Sci. (4)1:10.
ABSTRACT
The aim of the present study was to investigate the gross osteomorphometric, radiographic and histological developmental changes of long bones of the hind limb in prenatal Sokoto Red goat. Thirty accidental wasted fetuses from Sokoto metropolitan abattoir were collected and used for the study. They were grouped into three stages; first trimester: (1-51 days), second trimester (52-102 days) and third trimester (103-153 days). After fetal dissection, gross osteomorphometric measurements comprising bone length, mid diaphysial circumference, breadth of proximal and distal ends, were done on the long bones of the hind limb using a metre rule from each trimester. They were then exposed to radiographic beam using Power mobile X-ray machine (YSX100VET, China). Thereafter the bones were used for routine histology using Hematoxylin-Eosin stain (H&E). The results revealed that the gross osteometric parameters measured increased significantly (P<0.0001) with age. The ossification and mineralization began at the second trimester and continued throughout the stages as determined using X-rays and confirmed by histological investigations. It was concluded that, there was direct proportionality between development of long bones of the hind limb in Sokoto Red goat and their gestational ages. Also, ossification and mineralization started at second trimesters in long bones of the hind limb in the prenatal Red Sokoto goat.
KEYWORDS: Sokoto Red goat; Osteomorphometry; Radiography; Histology; Ossification; Trimester
INTRODUCTION
Goat are important sources of animal protein in the world, contributing to about 30 percent of the meat consumed, milk, cheese and other by-products such as hide and skin for leather production [1]. Previous study showed that goat has emerged as one of the major livestock species raised as compared to the sheep in Nigeria [2]. There are three distinct breeds of goats in Nigeria; West African Dwarf (WAD), Sokoto Red goat (SRG), and Sahel or desert [2] with Red Sokoto goats being common to the Northern region of the country [3]. Numerous studies on the endochondral ossification on long bone of several mammals were recorded in literature but there is a dearth of knowledge on the detail osteomorphometric, histological and radiologic sequences associated with long bone development. Miller, (1993) [4] divided bone development into two phases, prenatal phase which occur in-utero when bone tissue starts to form and postnatal phase which occur as the animal start to grow in which bone change in length and shape to assume adult form. Vaughan, (1980) [5] divided factors that influence bone growth into two main groups: Endogenous (genetic and hormonal) and Exogenous (environmental and dietary) factors. The process of bone formation is termed osteogenesis [6]. The initiation of skeletogenesis (Osteogenesis) starts with migration of mesenchymal cells to the sites of the future bones and condensation of the cells to form high cellular density that outline the shape and size of the future bones [7]. The mesenchymal cells either differentiate into chondrocytes and form cartilage models of the future bones (endochondral bone formation) or differentiate into osteoblasts to directly from bone (intramembranous bone formation) [7]. However, those that develop in fibrous tissue are called membrane bones, while those from cartilage are termed cartilage bones [7]. Longitudinal growth of long bones occurs at epiphysial plate through endochondral growth and ossification process [8]. The major determinants of longitudinal growth rate are chondrocytes proliferation rate, hypertrophy and bone matrix synthesis in the growth direction [9]. These factors are regulated by biological (genes, hormones, nutrients) and mechanical (load acting on the bone) factors [9]. Although, lifestyle and environmental factors affects the bone property such as cross-sectional geometry and compactness [10]. Long bones (Ossa longa) are predominantly found in limbs, with elongated cylindrical tubular part that encloses the medullary cavity containing medulla or marrow (diaphysis) and two extremities (epiphysis) associated with metaphysis connecting the later together [6]. In mammals, histologically bone exists in two distinct forms; woven bone and lamellar bone [11]. Woven bone (immature bone, fibrous bone, primary bone tissue) is most characteristically in the fetus and in the callus that is produced during fracture repair [11]. Macroscopically, lamellar bone exists in two forms; compact bone and trabecular bone [11]. Histologically, Bone is made up of specialized cells (osteoblasts, osteocytes and osteoclast) and a mineralized extracellular matrix [12]. The major product deposited into the bone matrix is a fibrous protein, collagen, which typically (although there is some variation) comprises about 70% of the matrix [12]. The aim of the present study therefore, is to investigate the gross osteomorphometric, radiographicand histological developmental changes of long bone of the hind limb in prenatal Red Sokotogoat.
Materials and Methods
Thirty (30) accidental wasted fetuses of the Sokoto Red goats were collected from the metropolitan abattoir, Sokoto, Nigeria, (at different gestational ages, over a period of two months) after obtainingapproval from the Institutional Animal Care and Use Committee (IACUC) of Usmanu Danfodiyo University, Sokoto (IACUC/UDUS/2019/AUP-RO44). The collected fetuses were fixed in 10% buffered formalin and then taken to the Veterinary Anatomy Laboratory of Usmanu Danfodiyo University, where the weight and age of the fetuses were determined. The fetal body weight was measured using electrical (digital) weighing balance for the smaller fetuses and compression spring balance (AT1422), size C-1, sensitivity of 20 kg X 50 kg for the bigger fetuses. The approximate age of the fetuses was estimated using the following formula as adopted by Dar et al. (2014) [13].
W 1/3 = 0.096 (t - 30)
Where, W= body weight of fetus in gram, t= age of the fetus in days
Based on the guide adopted by Bello A, et al. (2016) [14] fetuses were placed into various stages of gestational ages (trimester): - First trimester: 1-51 days (Early: 1 - 16, Mid: 17 - 34, Late: 35 - 51).Second trimester: 52 - 102 days (Early: 52 - 68, Mid: 69 - 86, Late: 87 - 102). Third trimester: 103 - 153 days (Early: 103 - 119, Mid: 120 - 137, Late: 138 - 153). Each of the fetal hind limb was detached from the coxo-femoral joints after placing the animal on lateral recumbency, by making incision using scalpel blade along the coxo-femoral joint and then rotated in opposite direction as earlier described by Sonfada ML, et al. (2015) [15]. A straight-line incision was made along the longitudinal axis of the limbs, the soft tissues on the limbs (fascia, muscles, tendons and ligaments) were removed, thus, the long bones were harvested. Gross osteomorphometric measurements comprising bone length, mid diaphysial circumference, breadth of proximal and distal ends, were done on the long bones of the hind limb using a metre rule from each trimester. This was achieved by taken the average of the circumference at proximal, middle and distal of the long bones [15]. Also, breadth of proximal and distal ends was measured using a metrerule. The dissected bones ranging fromfirst trimester (Late), second trimester (Early, mid and late) and third trimester (Mid) were exposed to radiographic beam using Power mobile X-ray machine (YSX100VET, China) at radiographic room of Veterinary Teaching Hospital, Usmanu Danfodiyo University Sokoto, at radiographic beam of 50 - 60 kV and 8 - 12 m. As recommended by Oviawe EI, et al. (2018) [16]. Individual bones were placed on the loaded cassette containing the X-ray film on the X-ray table and the light beam was collimated with the primary beam focused on the bones [16]. Upon exposure the cassettes were taken to the dark room for manual processing of the films in the processing tank after which they were allowed to dry. The X-ray films were viewed by placing them on X-ray film illuminator. Representative tissue samples from the samples fixed in 10% buffered formalin solution were taken from mid diaphysial parts of the long bones of hind limb at all trimesters for routine histology (H&E stains). The samples from the second and third trimester stages were decalcified as described by Bain, (2001) [17]. Samples were then dehydrated and embedded in paraffin wax. The 5 micrometer serial sections were mounted on glass slides and stained using hematoxylin-eosin stains. The slides were evaluated under a light microscope at lower and higher magnifications and micrographs were captured using Amscope camera 3.7 version (MU1000) 10.0 megapixels.
STATISTICS
All value variables are expressed as mean and standard deviation, while a G*Power 3.1 version (Germany) was used to estimate the samples size per gestational stage of with a minimal of n = 5 in each stage with a fixed effect size of 0.8. However, GraphPad PRISM 8.0 (GraphPad Software CA, USA) was used to run the descriptive statistics and analysis of variance between the lengths of the long bone of the fetal hind limb.
RESULT
Thirty accidental wasted fetuses were used (5 at first trimester, 20 at second trimester and 5 at third trimester.
Gross Osteomorphometry
The mean length of femur increased from 0.58 ± 0.08 cm at first trimester to 3.58 ± 0.66 cm at third trimester while the mean length of tibia increased from 0.76 ± 0.15 cm at first trimester to 4.70 ± 0.66 cm at third trimester which were all statistically significant (P < 0.0001) (Tables 1 and 2). There was a remarkable increase in the mean length of large metatarsal bone from 0.56 ± 0.08 cm at first trimester to 3.24 ± 0.76 cm at third trimester (Tables 1 and 2).The mean diaphysial circumferences of femur, tibia, large metatarsal increased from 0.5 ± 0.10 cm, 0.56 ± 0.05 cm, 0.47 ± 0.10 cm respectively at early second trimester to 1.45 ± 0.32 cm, 1.54 ± 0.17 cm, 1.27 ± 0.21 cm respectively in third trimester (Table 2). The mean breadths of proximal end of femur, tibia, large metatarsal increased from 0.26 ± 0.04 cm, 0.28 ± 0.04 cm, 0.19 ± 0.03 cm respectively at early second trimester to 0.88 ± 0.13 cm, 1.10 ± 0.18 cm, 0.74 ± 0.15 cm respectively at third trimester (Table 2). The mean breadths of distal end of femur, tibia, large metatarsal increased from 0.21 ± 0.05 cm, 0.20 ± 0.02 cm, 0.17 ± 0.02 cm respectively at early second trimester to 0.92 ± 0.22 cm, 0.88 ± 019 cm, 0.70 ± 0.23 cm respectively at third trimester (Table 2).
|
|
1st Trimester |
2nd Trimester |
3rd Trimester |
P value |
|
Limb Length (cm |
2.32 ± 0.28 |
|
|
|
|
Femur Length (cm) |
0.58 ± 0.08 |
2.60 ± 0.34 |
3.58 ± 0.66 |
<0.0001 |
|
Tibia Length (cm |
0.76 ± 0.15 |
3.33 ± 0.45 |
4.70 ± 066 |
<0.0001 |
|
Large Metatarsal Length (cm) |
0.56 ± 0.08 |
2.33 ± 0.20 |
3.24 ± 0.76 |
<0.0001 |
Table 1: Mean length of the long bones of fetal hind limb.
|
Bone |
Trimester |
|
Breadth of proximal end (cm) |
Breadth of distal end (cm) |
|
Femur |
Early second |
0.50 ± 0.10 |
0.26 ± 0.04 |
0.21 ± 0.05 |
|
|
Mid second |
0.77 ± 0.09 |
0.48 ± 0.07 |
0.43 ± 0.07 |
|
Late second |
1.04 ± 0.25 |
0.63 ± 0.05 |
0.83 ± 0.10 |
|
|
Third |
1.45 ± 0.32 |
0.88 ± 0.13 |
0.92 ± 0.22 |
|
|
Tibia
Large metartasal
|
Early second |
0.56 ± 0.05 |
0.28 ± 0.04 |
0.20 ± 0.02 |
|
Mid second |
0.82 ± 0.14 |
0.52 ± 0.08 |
0.40 ± 0.08 |
|
|
Late second |
1.13 ± 0.17 |
0.73 ± 0.15 |
0.56 ± 0.05 |
|
|
Third |
1. 54 ± 0.17 |
1.1 ± 0.18 |
0.88 ± 0.19 |
|
|
Early second |
0.47 ± 0.10 |
0.19 ± 0.03 |
0.17 ± 0.02 |
|
|
Mid second |
0.80 ± 0.11 |
0.35 ± 0.06 |
0.36 ± 0.07 |
|
|
Late second |
0.98 ± 0.09 |
0.53 ± 0.05 |
0.05 ± 0.00 |
|
|
Third |
1.27 ± 0.21 |
0.74 ± 0.15 |
0.70 ± 0.23 |
Table 2: Mean gross osteomorphometric parameters of second and third trimester hind limbs.
Radiographic findings
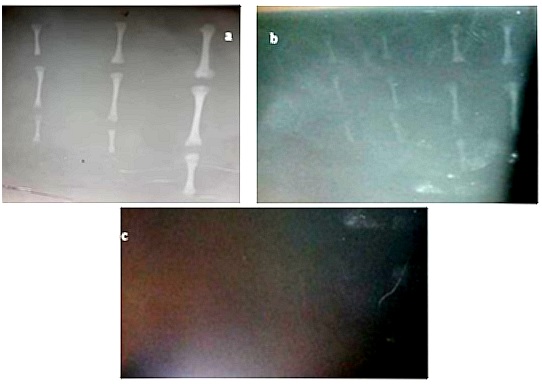
The radiographic image of femur, tibia and large metatarsal (proximal to distal) in third trimester (Mid) revealed that in all the three ages, the epiphysis and physis (growth plate) are less radiopaque, metaphysis moderately radiopaque while diaphysis is more radiopaque as shown in figure 1. In all the three ages radiopacity of epiphysis, physis, metaphysis and diaphysis of femur is marked, moderate in tibia, less in large metatarsal and it decreases down the ages also radiopacity decreases down the limb (Femur-tibia-large metatarsal) and ages as shown in figure 1a. The radiographic image of femur, tibia and large metatarsal (proximal to distal) in second trimester (Early, mid and late) revealed that radiopacity of epiphysis, physis (growth plate), metaphysis and diaphysis are less compared to those in third trimester as shown in figure 1b. Radiopacity decreases down the limb (femur-tibia-large metatarsal) and down the ages as shown in Figure 1b. The radiographic image of femur, tibia and large metatarsal (proximal to distal) in first trimester (Late) revealed that all the femur, tibia and large metatarsal are radiolucent as shown in figure 1c.
Figure 1: Radiographic image (a) Mid third trimester stage (b) Early, mid and late second trimester stage (c) Late first trimester stage hind limb long bones.
Histological findings
Transverse histological section revealed presence of chondrocytes in lacunae and presence of cartilage extracellular matrix in mid diaphysis of femur, tibia and large metatarsal at both late first and early second trimester stages as shown in figures 4a, 4b, 4c,4d, 4eand 4f respectively.
As from early second trimester, evidence of commencement of ossification and mineralization were observed in femur as shown in figure 4g. These, were observed all through the remaining stages due to the presence of osteocytes in lacunae and bone extracellular matrix that were observed as shown in figure 4h, 4i and 4j.
Figure 2: Photomicrograph section (Transverse section) (a) Femoral mid diaphysis at late first trimester stage showing Chondrocyte (Blue arrow), Lacuna (Black arrow) and Cartilage extracellular matrix (Red arrow). (b): Tibial mid diaphysis at late first trimester stage (c) Large metatarsal mid diaphysis at late first trimester stage. (d) Mid tibial diaphysis at early second trimester stage showing Chondrocyte (Blue arrow), Lacuna (Black arrow) and Cartilage extracellular matrix (Red arrow) (e) Mid femoral diaphysis at early second trimester stage (f) Large metatarsal mid diaphysis at early second trimester stage (g) Femoral mid diaphysis at early second trimester stage showing Bone extracellular matrix (start to form) (Blue arrow) (h) Femoral mid diaphysis late second trimester stage revealing the Osteocytes (Blue arrow), Lacuna (Green arrow), Haversian canal (Red arrow), and Bone extracellular matrix (Black arrow) (i) Tibial mid diaphysis at late second trimester stage (j) Large metatarsal mid diaphysis at late second trimester stage. H & E X400.
DISCUSSION
The osteometric results in this study showed an increase across the trimesters in all the parameters measured. The tibia was the longest bone with the highest mean length and highest mean diaphysial diameter as well as having the highest means value of diaphysial circumference. It is an established fact that bone growth in length and diameter depends primarily on the amount of calcium salt deposited during ossification [18]. This in turn depends on the quantity of this mineral in animal feed and the ability of the animal to use the mineral for bone calcification [19]. In the present study, the long bones of the hind limb in the second and third trimester stages were observed to possess typical characteristics of long bones with diaphysis connecting the two epiphyses. Proximal and distal epiphyses were observed to be cartilaginous with distinguishable features. This is in accordance to Shapiro S. (2008) [20] who reported that these regions are established by the middle of the embryonic stage and go through proportional changes in size until skeletal maturity. In the present study, there was no mineralization (cartilaginous) of the long bones of the hind limb in all the fetuses in the first trimester, as all the radiographs were completely radiolucent. However, at second and third trimester stages, they all appeared radiopaque indicating that there was mineralization, with radiopacity decreasing down the ages and proximodistally down the limb with the diaphysis appearing most radiopaque. This present result is line with the previous report of Ahmed, (2008), [21] that ossification of long bones begins in the diaphysis and sequence of appearance of the limb bones ossification occurs in proximodistal direction and it grew proximally faster than distally. Plain radiography relies on natural and physical contrast based on the density of material through which the X-ray radiation must pass [22]. Calcium is responsible for mineralization of bone thus increase in bone density. This helps to explain why there was no mineralization at first trimester. From the present study, ossification was observed to begin at second trimester stage of development. Presence of chondrocytes in lacunae and matrix on photomicrographs of mid diaphysis of first trimester stage bones proved that the bones are cartilaginous at that stage. In early second trimester there was evidence of ossification in femur due to presence of some mineralized extracellular bone matrix in some areas and this shows that ossification starts at second trimester stage. In late second trimester and third trimester the histological slides of mid diaphysis of the hind limb long bones proved that they are ossified due to presence of osteocytes in spaces known as lacunae and mineralized extracellular matrix. Our histological result is backing our gross result that says, first trimester stage bones are not ossified, and ossification starts at second trimester stage. The present result is in line with previous reports of report of Ahmed (2008) [21], who said the primary ossification centers in the forelimb bones of sheep appeared in a limited period range from 43-56 days old in the studied fetuses, but the centers of ossification in carpal bones delayed to appear till 90-108 days old.
CONCLUSION
The current study described the main changes in osteomorphological, histological and radiological event associated with development of the long bone of the hind limbs of the Sokoto Red Goat, which reveal development changes increase with age, also ossification and mineralization start at second trimester stage in long bones of the hind limb in the prenatal Red Sokoto goat. Therefore, apart from the contribution of the present study to the knowledge of anatomy for this breed and or species, it also provides a baseline for comparison with other breed or specie. The results presented in this study could be considered in future molecular studies of endochondral bone formation in long bone of the hind limbs.
Acknowledgments The authors wish to acknowledge the support of the Gross and Histology Unit, Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto-Nigeria, for their assistance during the analysis.
Author Contribution
DA, FAK and NA substantial contributions to the conception, FAK and NA sampling and dissection. DA, ASM and BA interpretation of data; DA and MMA rafted the work and AK, DA and MMM substantively revised the manuscripts and approved the submitted version and also agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
FUNDING
No funding was obtained for this study.
AVAILABILITY OF DATA AND MATERIAL
The datasets used and/or analysed during the current study are available with the corresponding author {Dr. Danmaigoro Abubakar} on reasonable request.
COMPETING INTEREST
The authors declare that they have no competing interests
REFERENCES
- Egbuhelu C, Eze EC, Ajibo F. (2018). Assessment of the Problems of Sheep and Goat Production, in Udi Local Government Area, Enugu State of Nigeria. IOSR Journal of Humanities and Social Science (IOSR-JHSS). 23(10):73-80.
- Anaeto M, Adeyeye J, Chioma G, Olarinmoye A, Tayo G. (2010). Goat products: Meeting the challenges of human health and nutrition. Agriculture and Biology Journal of North America. 1(6):1231-1236.
- Lawal-Adebowale OA. (2012). Dynamics of ruminant livestock management in the context of the Nigerian agricultural system. Livestock production. 4:1-20.
- Miller ME. (1993). Millers anatomy of the dog (3rd ed) W.B. Saunders company. pp. 45, 126.
- Vaughan JM. (1980). The Physiology of Bone. Oxford University press London. New York. 158-165.
- Setiawati R, Rahardjo P. (2018). Bone Development and Growth. In Osteogenesis and Bone Regeneration. Intech Open. DOI: 10.5772/intechopen. 82452.
- Berendsen AD, Olsen BR. (2015). Bone development. Bone. 80:14-18.
- Atabo SM, Umar AA, Shehu SA, Yakubu AS. (2018). Epiphyseal plate closure of radio-ulna bone in red Sokoto goat ecotype. Sokoto Journal of Veterinary Sciences. 16(1):71-78.
- Yadav P. (2015). Modelling loading and growth of long bones Modelling loading and growth of long bones (Doctoral dissertation, KTH Royal Institute of Technology). 23-94.
- Straehl FR, Scheyer TM, Forasiepi AM, MacPhee RD, Sánchez-Villagra MR. (2013). Evolutionary Patterns of Bone Histology and Bone Compactness in Xenarthran Mammal Long Bones. PLoS ONE. 8(7):e69275.
- Martiniaková M, Omelka R, Chrenek P. (2010). Compact bone structure of unmodified and genetically modified rabbits. Slovak Journal of Animal Science. 43(3):166-171.
- Brian K. Hall (2013). The embryonic development of bone. American Scientists, 72(2):174-181.
- Dar Y, Sarma K, Suri S, Devi J. (2014). Histochemical Studies on the Tongue of Goat Foetii (Capra hircus). Indian Journal of Veterinary Anatomy. 26(2):99-100.
- Bello A, Dabai DI, Umaru MA, Shehu SA. (2016). Histomorphometric study on fetal development of red sokoto goat kidney. Direct research journal of Agriculture and food science. 4(4):66-74.
- Sonfada ML, Shaibu MA, Hena SA, Danmaigoro A. (2015). Morphologic and Morphometric Studies of Long Bones of One-Humped Camel Fetuses. Sudan Journal of Science and Technology. 16(3):21–28.
- Oviawe EI, Yakubu AS, Kene ROC, Shehu SA. (2018). Radiographic Evaluation of the Appearance and Closure Time of Growth Plates of Radius and Ulna Bones in Nigerian Indigenous Dogs. J Vet Ani Res. 1:101.
- Bain BJ. (2001). Bone marrow trephine biopsy. Journal of clinical pathology. 54(10):737-742.
- Richardson C, Herbert CN, Terileki S. (1976). Estimation of the developmental age of the ovine foetus and lamb. Veterinary Record. 99:22-26.
- Sivachelvan MN, Ali MG, Chibuzo GA. (1996). Foetal age estimation in sheep and goats. Small Ruminant Research. 19(1):69-76.
- Shapiro S. (2008). Bone development and its relation to fracture repair. 10The role of mesenchymal osteoblasts and surfaceosteoblasts. European Cells and Materials. 15:53-76.
- Ahmed SN. (2008). Development of forelimb bones in indigenous sheep fetuses. Iraqi Journal of Veterinary Science. 22(2):87-94.
- Chen YM, Pope LT, Ott JD. (2010). Basic radiology (2nd ed.): the macgrew-hill education/medical. p1-5.