Information Links
Related Conferences
Previous Issues Volume 9, Issue 10 - 2024
Peritonitis Secondly to the Hepatocolic Schistosomiasis
Niyirera E1,*, Fatmata Yabome S2, Boima F3
1Department of Surgery, Koidu Government Hospital, Sierra Leone
2Department of Surgery, Connaught Referral Hospital, Sierra Leone
3Research Unit, PIH, Sierra Leone
*Corresponding Author: Niyirera Eugene, Department of Surgery, Koidu Government Hospital, Sierra Leone, Phone: +250788853948, Email: [email protected]
Received Date: October 08, 2024
Published Date: November 06, 2024
Citation: Niyirera E, et al. (2024). Peritonitis Secondly to the Hepatocolic Schistosomiasis. Mathews J Case Rep. 9(10):190.
Copyrights: Niyirera E, et al. (2024).
ABSTRACT
Background: Schistosomiasis is a chronic parasitic infection endemic in many countries. Colonic schistosomiasis is a rare entity with no specific clinical manifestations or endoscopic aspects, which delay the diagnosis. Diagnosis is primarily dependent on histopathological analysis, and treatment with antihelminthics typically resolves the infection. Case Summary: A 17 years old male patient who consulted emergency department with features of chronic malnutrition and generalized peritonitis associated with hepatomegaly. Lab investigations have shown anemia and leucocytosis. Emergency laparotomy was done and found granulomatous distal colon from rectum to the splenic flexure, multiple mesenteric lymph nodes and hepatosplenomegaly with multiple nodules on the liver. Biopsies for lymph node and from sigmoid were taken then abdomen was closed. Perioperatively, was given antibiotics (ceftriaxone and metronidazole) and discharged at POD7. Was seen again after 2 months after getting histology results. Biopsy was taken and revealed dead and live schistoma and inflammatory mesenteric lymph nodes. The boy has been reassessed was gaining weight, no complain, no organomegaly. The patient was having been given Praziquantel. Conclusion: Colonic schistosomiasis may cause peritonitis due to reactive inflammatory mesenteric lymph nodes and colonic granuloma, exploratory laparotomy or diagnostic laparoscopy and tissue biopsy confirm the diagnosis. Praziquantel may be initiated as long as schistosomiasis is highly suspected.
Keywords: Intestinal Schistosomiasis, Endemic, Neglected Tropical Diseases.
Schistosomiasis is a chronic parasitic disease caused by a trematode bloodfluke of genus schistosoma that belongs to the schistosomatidae family [1,2]. It is a multifocal disease that includes environmental, behavioral, parasitic, vector and host factors [2-4]. 5 types. S. intercalatum occurs in central and west Africa [4,5]. Hepatic schistosomiasis is the most common form of the chronic disease and usually results from heavy S. mansoni infection [6]. Intestinal schistosomiasis represents another form of schistosomal affection. Among spectrum of intestinal lesions, polyps are the commonest [7,8]. In the large intestine, ova are mainly distributed in the loose submucosa, and to a lesser extent in the subserosa where infrequently multiple granuloma are formed. Sandy patches develop when the submucosa becomes densely thickened by fibrous tissue containing immense numbers o of calcified eggs; the overlying mucosa becomes atrophic and acquires a granular dirty yellowish appearance [9,10]. Schistosomal colonic polyposis affects mainly adult males [11].
CASE REPORT
A 17 –years-old male presented to the emergency department with severe abdominal pain, cough and fever for 7 days.
He reported no relevant medico surgical history but comes in Tonkolili district of Sierra Leone, one of endemic zones of Schistosomiasis. On physical examination vitals were as follow: temperature 39 degree Celsius, heart rate 26 cycles per minute, heart rate 128 beat per minute, Oxygen saturation 89%, blood pressure 91/56 mmHg. Was chronically malnourished, having jaundice, on chest was symmetric with good air entry, abdomen was slightly distended, decreased bowel sounds, generalized tenderness, guarding and huge hepatomegaly. On DRE rectum was full of soft stool. Our impression was generalized peritonitis secondly to the hollow viscus perforation.
Management plan was to do radiological and lab investigations, resuscitation antibiotics, painkillers and preparation for emergency exploratory laparotomy.
Full blood count revealed: Hemoglobin: 5.9g/dl, WBC: 14.4x103/ml, platelets: 317x 103/ml. Abdominal Ultrasound revealed free fluids in the peritoneal cavity and hepatosplenomegaly. Abdominal X ray did not reveal free air in the abdomen.
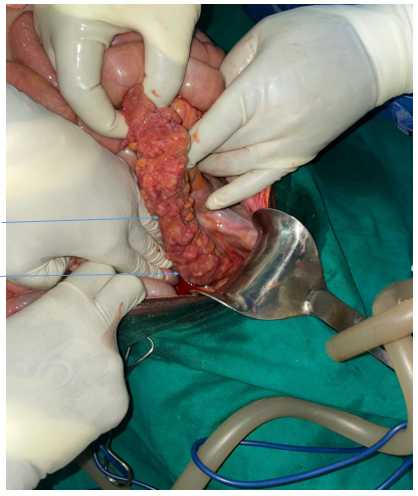
The patient was taken to the operating room and under general anaesthesia laparotomy was done. Intraoperatively we found minimal inflammatory fluids, multiple mesenteric lymph nodes, from the rectum to the splenic flexure the colon was having multiple friable nodules matted together, there was no obstruction Picture (…). The liver was enlarged with multiple nodules, the spleen was slightly enlarged. We took biopsy from mesenteric lymph nodes and colon, did washout and closed abdomen. Postoperatively the patient continued antibiotics (ceftriaxone and flagyl) and tramadol for 5 days, improved well and has been discharged on POD 6.
After 2 months, histology analysis revealed loose mesenchymal tissue with intense chronic inflammation and neovascularization. There are numerous foci of dead and viable helminthic organisms (schistosoma) with attendant strong granulomatous inflammatory reaction and reactive fibrosis. The accompanying lymph node shows only reactive changes of sinus histiocytosis. No evidence of malignancy.
The boy has been called for follow up, was healthy without complain, was getting weight, physical exam was normal, no more hepatomegaly. As he was coming from endemic area we gave him Praziquantel.
Picture taken intraoperatively showing granulomatous pattern of rectosigmoid (1. Glanulomatous rectum, 2. Glanuromatous sigmoid)
DISCUSSION
Proctocolitis due to schistosomiasis is a rare disease with very limited literature [9]. Chronic schistosomiasis results from the eggs logging, inducing the inflammation, granuloma formation, fibrotic changes. In the colon, the shed eggs, penetrate the bowel adjacent to the mesenteric vessel, whereas unshed eggs go back to portal circulation lodge and induce granulomatous reaction. In the liver the chronic progression causes periportal fibrosis known as Symmers piperistem fibrosis [12].
Clinical manifestations of intestinal schistosomiasis differ from one patient to another based on the disease process and incidence of complications [13]. In the endemic area of Schistosomiasis, the stool examination is the main stay for diagnosis. Negative stool examination does not always exclude Schistosomiasis, has low sensitivity and fluctuation in shedding of eggs. Molecular test to detect Schistosoma DNA in fecal specimen shows the greater sensitivity compared to stool microscopy. Biopsy as well has high sensitivity. Antibody testing also has a high sensitivity but its use is in endemic zones since antibodies remain detectable after parasitological cure. Antigenes can be detected and quantified. The direct fecal smear also known as wet- mount preparation is done for detection of protozoa and helminths [14]. In the endemic region in a patients presenting with features of peritonitis in the limited resource settings, to get biopsy results it takes long. In young adults where the index of suspicion of colon cancer is low [15], intestinal schistosomiasis should be highly suspected.
Praziquantel is the standard treatment of choice for schistosomiasis. Artemisin derivates are also effective in cericariae and can be used in conjunction praziquantel to improve infection control [16]. The initial inflammatory response is reversible but collagen deposition and fibrosis is partial reversible. The granulomas may disappear after adequate treatment.
CONCLUSION
This case of rectosigmoid schistosomiasis emphasizes the need for heightened clinical awareness, timely diagnosis, and cautious surgical decision making in endemic regions. It serves as reminder of the importance of considering schistosomiasis in the differential diagnosis of gastrointestinal symptoms and support the use of biopsy to guide the treatment.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
REFERENCES
- McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. (2018). Schistosomiasis. Nat Rev Dis Prim. 4(1):13.
- Yuan Y, Zhao J, Chen M, Liang H, Long X, Zhang B, Chen X, Chen Q. (2021). Understanding the Pathophysiology of Exosomes in Schistosomiasis: A New Direction for Disease Control and Prevention. Front Immunol. 12:634138.
- Rivadeneira D, Luo H. (2018). Intestinal Schistosomiasis Caused by Schistosoma japonicum: A Literature Review. J Infect. 2(1):9-13.
- Olveda DU, Li Y, Olveda RM, et al. (2013). Tropical Medicine & Surgery Bilharzia : Pathology , Diagnosis , Management and Control. Trop Med Surg. 1(4):1-9.
- CambridgeCore_Citation_07Jun2024 (1).
- Liu Z, Zhang L, Liang Y, Lu L. (2022). Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis. Front Cell Infect Microbiol. 12:1035765.
- Rivadeneira DJ,Luo HS. (2019). Intestinal Schistosomiasis Caused By Schistosoma japonicum: A literature review. J of Infectiology. 2(1):9-13.
- Mengistu M, Shimelis T, Torben W, Terefe A, Kassa T, Hailu A. (2011). Human intestinal schistosomiasis in communities living near three rivers of jimma town, South Western ethiopia. Ethiop J Health Sci. 21(2):111-118.
- D'Souza K, Birnie BW, Garraway N. (2020). Large bowel obstruction secondary to schistosomiasis-related colonic stricture. Int J Surg Case Rep. 74:209-213.
- Wang C yu E, Bobrow JE, David J, Thorpe SKS, Li Y, Crompton RH. D ik = ∑ Tr ( U. Published online 2007:249-254).
- Bosire F, Orwa I, Mwachiro M, Parker R, White R, et al. (2018). Scistosoma Mansoni Proctocolitis with Polyposis. annals of African Surgery. 15(2).
- Llanwarne F, Helmby H. (2021). Granuloma formation and tissue pathology in Schistosoma japonicum versus Schistosoma mansoni infections. Parasite Immunol. 43(2):1-12.
- Qin X, Liu CY, Xiong YL, et al. (2021). The clinical features of chronic intestinal schistosomiasis-related intestinal lesions. BMC Gastroenterol. 21(1):1-11.
- Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S. (2015). New diagnostic tools in schistosomiasis. Clin Microbiol Infect. 21(6):529-542.
- Ferrari A, Bertario L, Signoroni S, et al. Colorectal Carcinoma in Children and Adolescents Lead contributors. p. 1-21. Available at: https://www.cure4kids.org/private/oncochap/ocrev_302/Onco-Ch68-Colorectal-Carcinaoma.pdf
- Abaza S. (2015). Treatment of Schistosomiasis: From Praziquantel to Development of New Drug Targets Treatment of Schistosomiasis. 6(1687-8942):2.