Previous Issues Volume 6, Issue 2 - 2024
Nephron Sparing Surgery for Large Renal Angiomyolipoma: A Case-Series and Literature Review
Rawan Alyousef1, Nesef Alnesef1, Salah Termos2,*, Abdullatif Alterki1, Reem Alshahabi1,2, Talal Alqaoud1
1Department of Surgery, Urology Unit, AlAmiri Hospital, Kuwait
2Department of Surgery, Hepatobiliary Unit, AlAmiri Hospital, Kuwait
*Corresponding author: Salah Termos, MD, FACS, Department of Surgery, Hepatobiliary Unit, AlAmiri Hospital, Kuwait, E-mail: [email protected]
Received Date: November 05, 2024
Published Date: December 13, 2024
Citation: Alyousef R, et al. (2024). Nephron Sparing Surgery for Large Renal Angiomyolipoma: A Case-Series and Literature Review. Mathews J Urol Nephrol. 6(2):23.
Copyrights: Alyousef R, et al. © (2024).
ABSTRACT
Introduction: Renal angio/myo/lipoma (AML) is a benign mesenchymal tumor with high bleeding potential and minimal risk of malignant transformation. Larger size masses can lead to serious medical conditions. Treatment usually varies from case to case ranging from minimally invasive approaches to surgical interventions. Objective and Methodology: The aim of this study is to endorse nephron-sparing surgery as an effective treatment option for large renal angiomyolipoma in facilities where intervention radiology is not readily available. In our manuscript we present a series of two cases of AML (above 8cm) treated surgically with open partial nephrectomy, attempting to achieve a shorter vascular exclusion time. Results: Large AMLs may pose challenges in management, with a focus on preserving renal function, using the minimally invasive approaches and minimizing the risk of life-threatening hemorrhage. Our case-series of nephron-sparing surgery (NSS) attaining short warm and cold ischemia times demonstrated a good outcome with acceptable complications. Both patients had an uneventful early postoperative course with normal creatinine and were discharged a few days after surgery. Only one patient had a late complication at two months, manifested by urinoma, which was managed with ureteral double-J stenting and percutaneous drain insertion. Otherwise, two years follow-up showed no abnormal laboratory or radiologic findings. Conclusion: Management of large renal angiomyolipomas can be challenging especially in places where embolization is not feasible and robot-laparoscopic modalities are not available. Therefore, open nephron-sparing surgery can be indicated as a safe and valid therapeutic choice with desirable outcomes.
Keywords: Renal Angiomyolipoma (AML), Nephron Sparing Surgery (NSS), Selective Angioembolisation (SAE).
INTRODUCTION
Renal angiomyolipoma (AML) is a rare type of benign solid renal neoplasm consisting of mature adipose tissue, dilated blood vessels, and smooth muscle. Angiomyolipoma has a prevalence of 0.2%-0.6%, with a female predominance of 2:1. It is Usually discovered incidentally between 50 and 60 years of age [1,2].
AML exists in three different variants: classic, epithelioid, and angiomyolipoma with epithelial cysts. The classic variant is the most common [3]. Apart from the sporadic etiology, which occurs in 80% of cases, AML is also known to occur in patients with tuberous sclerosis complex (TSC) and pulmonary lymphangioleiomyotosis (LAM) [4].
AML is classified by size as small (<4 cm), medium (≥4-8 cm), and large/giant (>8cm). Approximately 30% of large AMLs are likely to be symptomatic and should be managed accordingly, as the risk of bleeding and hemorrhagic shock is substantial (10-15%) compared to 2% in small AMLs [5,6]. Various treatment modalities exist to manage significant sized AML (>4cm) including: selective renal angioembolization (SEA), radiofrequency ablation (RFA), nephron sparing surgery (NSS)/partial nephrectomy (PN), and radical nephrectomy. We report two cases of large AML managed by open nephron sparing surgery with good outcomes.
CASE PRESENTATION
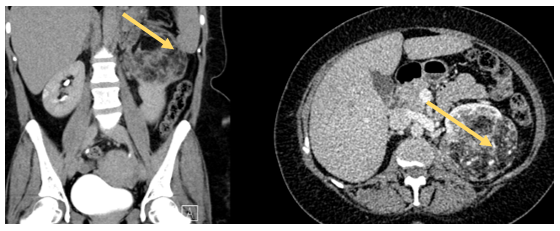
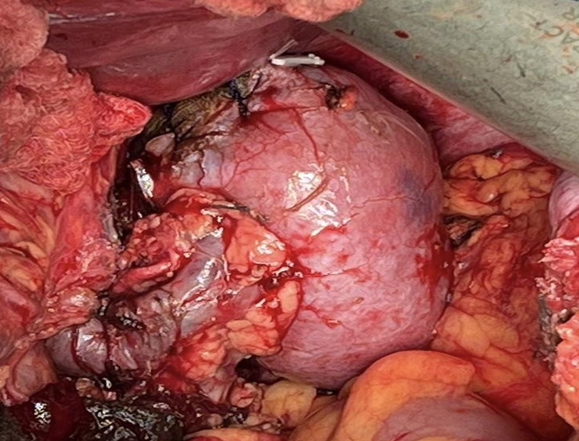
Case One: A 70-year-old female patient, previously healthy presented with symptoms of nausea, vomiting, and abdominal pain. She underwent a CT scan of the abdomen and pelvis, which revealed right renal AML measuring 10x7x6 cm, with a 2 cm Bosniak 1 cyst in the lower pole of the right kidney (Figure 1). She had a normal contralateral kidney, and a normal baseline creatinine (0.8 mg/dL) and eGFR (>60). The patient was counseled on her diagnosis and advised to purse active management rather than surveillance. Given the lack of an interventional radiologist readily available for angioembolization, the option of surgery (open partial nephrectomy) was discussed with the patient, and she agreed to proceed. The operation started with insertion of a 24 cm 6F double J ureteral stent into the right kidney. A Right sided Makuuchi incision was made (Figure 2) and a Bookwalter retractor system was deployed. The hepatic flexure was lowered, and kocherization of the duodenum was performed. The kidney was carefully mobilized, and the renal hilum was then dissected to identify the renal artery and vein. The AML was then carefully dissected at its base on the parenchyma, and using intraoperative ultrasonography the parenchyma was scored with electrocautery to delineate the resection margins. Clamping of the renal artery and vein was achieved followed by the application of sterile ice to achieve warm and cold ischemia (total ischemia time 32 min) after the administration of IV mannitol. The tumor was then entirely resected (Figure 3), and hemostasis was achieved. The calyceal defect was closed individually with 5-0 PDS, sutures renorrhaphy was then completed in two layers with bolstering sutures in a horizontal mattress technique using 2-0 PDS with site Surgicel®. An omental patch was buttressed over the resection, and a 19F JP drain was placed in the perinephric area. Estimated blood loss was 200cc.
Figure 1. Large AML Rt Kidney.
Figure 2. Makucchi Incision in complex urology cases.
Figure 3. Gross appearance of the Rt kidney tumor before and after resection.
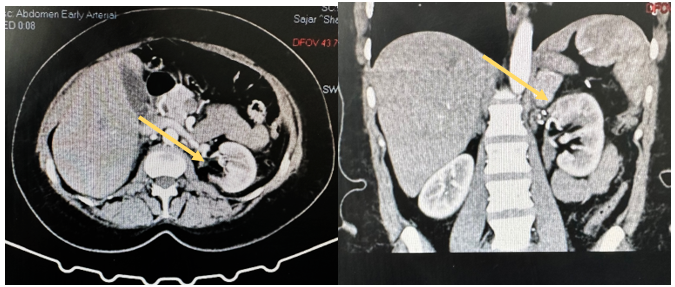
Her hospital course was unremarkable, and she was discharged on postoperative day 6. Prior to drain removal, creatinine level was checked and was negative for a urine leak. She was then followed up in the outpatient department(OPD), and the with a final pathological report revealed a classic AML with negative margins. The ureteral stent was removed 6 weeks later. On two-years-follow up, her creatinine level was (0.81 mg/dL) and a CT scan demonstrated complete resection of the AML with expected postoperative changes (Figure 4).
Figure 4. CT scan showing complete resection of Rt kidney AML.
Case Two: A 46-year-old female with a history of sleeve gastrectomy, chronic anemia, and hypertension was referred to us for evaluation of left-sided flank pain. Her last imaging study, conducted 9 years ago, showed a 3 cm left renal AML. A CT scan of the abdomen and pelvis revealed a 10x9x8.3 cm lesion in the left upper pole, suggestive of AML (Figure 5). A coinciding right non-obstructing 9 mm renal stone was noted in her malrotated right kidney. The patient was counseled on her diagnosis and advised to undergo active management. Due to the limited availability of interventional radiologists and the absence of robotic services, the patient was offered an open nephron-sparing vs radical nephrectomy and she agreed on proceeding with surgical intervention. Given the floating stone in her right kidney and the planned complex partial heminephrectomy on her left kidney, we inserted bilateral double J stents at the beginning of her surgery. A left sided modified Makuuchi incision was performed, the abdomen was entered and the left colon was mobilized along with a medial visceral roll reflecting the spleen, pancreas, and stomach away from the left retroperitoneum. The omni retraction system was then deployed and the kidney was carefully dissected circumferentially. The renal hilum was dissected with subsequent exposure of the renal artery and vein. Intraoperative ultrasound was used to identify the margins around the lesion in the upper pole prior to dissection. Warm and cold ischemia was performed (total time 28 min), and the upper pole lesion was resected. Bleeding was encountered during the resection with an estimated blood loss of 300 ml. Tumor resection was then completed (Figure 6), and the encountered vessels were ligated individually with 5-0 Prolene. Calyceal defects were closed individually with 5-0 PDS, the renorrhaphy was completed in two layers with bolstering sutures in a horizontal mattress manner using 2-0 PDS with Surgicel®. After renorrhaphy and securing hemostasis, nephropexy to the lateral wall and an omental wrap over the resected kidney was performed, and a 19F JP drain was placed. The patient was discharged on post-operative day 7, with her drain removed after demonstrating a normal creatinine level. She was followed up at the OPD, and the pathology report showed classic AML. Her double J stent was removed 6 weeks after surgery with right sided ureteroscopy and stone lithotripsy. The procedure was uneventful and both stents were removed successfully.
Figure 5. CT Imaging demonstrating a large Lt Kidney AML.
Figure 6. Large Lt Kidney AML.
Two months after surgery, the patient presented with flank pain and fever. CT urography demonstrated a 5 cm urinoma and evidence of a urine leak. She underwent percutaneous aspiration of the urinoma, retrograde double-J stent insertion and a urethral catheter. Four weeks later, a follow-up ultrasound revealed a complete resolution of the urinoma, and a retrograde study showed no leakage; therefore, her stent and urethral catheter were removed. At the two-year-follow-up, her creatinine level was normal (0.7 mg/dL), and CT demonstrated complete resection of the AML and a well-perfused kidney (Figure 7).
Figure 7. Imaging Post resection of Lt AML.
DISCUSSION
Angiomyolipoma is the most common benign kidney tumor. It has a primarily sporadic occurrence in most cases, whereas 20% have a direct association with tuberous sclerosis complex. Renal angiomyolipoma occurs in 70-80% of patients afflicted with TSC. When compared with sporadic AML cases, tumors in TSC patients are more likely to be larger in size, bilateral and multifocal, have a more rapid growth rate, and tend to present with AML at a younger age [7,8].
They are typically diagnosed as an incidental finding via imaging studies; however, flank pain and hematuria may also be a common presentation. Large angiomyolipoma may rarely present, in the form of life-threatening hemorrhage known as Wunderlich’s Syndrome, which can present with life-threatening shock [4]. Management of AML depends on the etiology (TSC-associated vs. sporadic), size, presence of symptoms, patient stability, women of childbearing age, and the presence of intra-tumoral aneurysm. In case of a small (< 4 cm) AML, active surveillance is advised, especially in sporadic cases. Symptomatic TSC-associated AML greater than 3 cm in size can be treated with mTOR inhibitors as first-line treatment.
Treatment of these mesenchymal tumors typically varies according to size, feasibility and expertise. They are usually managed by ablation, embolization or resection. Surgical resection can be partial or complete, laparoscopic or robotic, transperitoneal or retroperitoneal. All these modalities are effective depending on the institution’s facilities and surgeon’s experience [9].
The benign nature of these lesions has led physicians to favor less aggressive approach like SAE and NSS [9]. This report describes two cases of large angiomyolipoma that were managed with an open NSS and followed for two years. With only one case complicated by urinoma at two months post-operative. Selective angioembolization (SAE) is recommended as first-line treatment in cases larger than 4 cm tumor or intra-tumoral aneurysm ≥ 5mm and bleeding AML [10-12]. SAE has the advantage of early recovery, decreased intra-operative blood loss, and shorter hospital stays; however, the issue of recurrence and the need for repeated intervention is a major concern, mainly in facilities where interventional radiology is not always feasible. A study by Kothary et al., in which 9 patients with AML larger than 4 cm underwent angioembolization as a primary treatment modality, revealed an overall recurrence rate of 31.6%, with 6 out of 10 patients with TSC experiencing recurrence. Similarly, a study by Nozadze et al. reported a 10-year experience with 49 patients with AML who had undergone SAE and reported a re-intervention rate of 20.4% [13, 14]. In a study by Bardin et al., 4 out of 23 patients required additional procedures due to recurrence following SAE, further highlighting the recurrence rates that have been reported variably in the literature [15].
On the other hand, NSS is also considered a valid therapeutic option that has consistently shown markedly reduced recurrence rates of AML when compared to angioembolization [16]. Boorjian et al. reviewed cases of AML managed by open PN. They reported a recurrence rate of 3.4% and a reintervention rate of 12% [17]. Another long-term follow-up study revealed no recurrence of AML in 28 patients treated with NSS [18]. The feasibility and safety of nephron-sparing surgery have been demonstrated in previous studies notably that done by Yip et al., where by a total of 23 patients with a mean tumor size of 12.3cm and a range of 1.5-30 cm were documented to have undergone nephron-sparing surgery for symptomatic renal AML with favorable results [19]. Nephron-sparing surgery also has the advantage of preserving renal function [20]. Early complications associated with partial nephrectomy most commonly include ileus (8.6%), urine leak (5%), and hemorrhage (1.7%).
Overall, nephron-sparing surgery remains one of the most commonly reported procedures for AML via open, laparoscopic and robotic surgeries. It is considered safe and yield a low recurrence rate [21,22]. These findings have been consistent with our case report having acceptable outcomes in both cases. Table 1 describes and compares various studies done on management of AML along with their follow-up.
Larger AMLs bleed more than smaller AMLs with reported malignant transformation into a sarcoma [23,24]. Giant AML may constitute serious sequelae when left untreated.
Contemporary studies have reported the constraints of open NSS regarding patient recovery time compared to minimally invasive techniques. Even though surgical approaches have a higher risk of bleeding and morbidity, they are considered more definitive and have a superior outcome with recurrence rate when compared to selective angioembolization alone [25]. Our cases were challenging ones, massive tumors, and we excluded the SAE and minimally invasive surgical options due to the absence of services and lack of expertise. We opted for an open NSS technique with short warm and cold ischemia time, achieving positive long -term end results.
Table 1. Comparison of studies regarding management of AML
|
Author |
Year |
Intervention |
No. of cases |
Mean size of mass |
Follow-up |
No of procedures on average |
|
Alyousef et al |
2024 |
Nephron-sparing surgery |
2 |
10 cm |
24 months complete resolution of the tumor with preserved kidney function. No recurrence noted. |
1 |
|
Andersen et al [21] |
2015 |
Angioembolization |
8 |
6.5 cm |
64% decrease in tumor size. Two patients passed away during follow-up of the patients (4.5 year follow up). 3 of the 8 patients lost a kidney during follow up, 1 of whom due to an iatrogenic injury caused by angioembolization. No recurrence of the AML noted by the researchers. |
8 |
|
Patatas et al [19] |
2013 |
Angie-embolization (using coils) |
13 |
6.2 cm |
1 of the 13 patients experienced minimal reduction in tumor size (by 6.5%) after 1 year follow up and required secondary angioembolization. The same patient experienced regrowth of AML on long term follow up. Two patients experienced hemorrhage requiring repeat angioembolization. |
16 |
|
Faddegon et al [20] |
2011 |
Angioembolization |
17 |
5.85 cm |
5 of the 17 patients required repeat angioembolization. 50% of the 17 patients experienced acute hemorrhage. |
22 |
|
Faddegon et al |
2011 |
Surgery (radical and partial nephrectomy) |
42 |
5.85 |
None of the 42 patients required any secondary procedures or experienced recurrence. 14% of the patients treated surgically experienced limited hemorrhage. |
42 |
|
Bishay et al [18] |
2009 |
Angioembolization |
16 |
15 cm |
2 of the 16 patients required repeat angioembolization due to recurrence when seen during long term follow up. One of the 16 patients had a hemorrhagic transformation of AML. |
10 of the 16 patients were treated in 1 session. The 6 others were treated over multiple sessions. |
|
Ramon et al [22] |
2009 |
Selective angioembolization |
41 |
10.3 cm |
No documented hemorrhage after long term follow up. 6% of the study required to undergo subsequent surgical management. |
41 |
|
Boorjian et al [1] |
2007 |
Nephron-sparing surgery |
58 |
3.9 cm |
2 patients have documented radiographic yet asymptomatic recurrence after a median postoperative follow up of 8 years, A urine leak was documented in 3 patients. No chronic insufficiency in any of the patients noted including in 4 patients who had a solitary kidney. |
58 |
Giant renal angiomyolipoma is an uncommon renal pathology that poses life-threatening complications. The management of larger tumors can be challenging due to many factors especially in facilities where angioembolization is not technically feasible and robotic laparoscopic modalities are not available. Hence, open nephron-sparing surgery can be considered as a safe and valid therapeutic choice with desirable outcomes in-terms of preserved renal function and lower recurrence rates.
ACKNOWLEDGEMENTS
The Authors thanks for Dr. Saba Altamimi for her illustrations in Figure 2.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING STATEMENT
No funding was received to assist with the preparation of this manuscript.
REFERENCES
- Vos N, Oyen R. (2018). Renal Angiomyolipoma: The Good, the Bad, and the Ugly. J Belg Soc Radiol. 102(1):41.
- Mauermann J, de Martino M, Waldert M, Haitel A, Klingler HC, Remzi M, et al. (2013). Gender differences in benign renal masses. World J Urol. 31(5):1051-1057.
- Aydin H, Magi-Galluzzi C, Lane BR, Sercia L, Lopez JI, Rini BI, et al. (2009). Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol. 33(2):289-297.
- Shamam YM, Leslie SW. (2023). Renal Angiomyolipoma. StatPearls Publishing.
- Dickinson M, Ruckle H, Beaghler M, Hadley HR. (1998). Renal angiomyolipoma: optimal treatment based on size and symptoms. Clin Nephrol. 49(5):281-286.
- Guo Y, Kapoor A, Cheon P, So AI, Lattouf JB, Jamal M. (2020). Canadian Urological Association best practice report: Diagnosis and management of sporadic angiomyolipomas. Can Urol Assoc J. 14(11):E527-E536.
- Bausch K, Wetterauer C, Diethelm J, Ebbing J, Boll DT, Dill P, et al. (2021). Enhancing disease awareness for tuberous sclerosis complex in patients with radiologic diagnosis of renal angiomyolipoma: an observational study. BMC Nephrol. 22(1):47.
- Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, et al. (2008). Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology. 72(5):1077-1082.
- Hughes-Hallett A, Patki P, Patel N, Barber NJ, Sullivan M, Thilagarajah R. (2013). Robot-assisted partial nephrectomy: a comparison of the transperitoneal and retroperitoneal approaches. J Endourol. 27(7):869-874.
- Vaggers S, Rice P, Somani BK, Veeratterapillay R, Rai BP. (2021). Evidence-based protocol-led management of renal angiomyolipoma: A review of literature. Turk J Urol. 47(Supp. 1):S9-S18.
- Hatano T, Egawa S. (2020). Renal angiomyolipoma with tuberous sclerosis complex: How it differs from sporadic angiomyolipoma in both management and care. Asian J Surg. 43(10):967-972.
- Krueger DA, Northrup H; International Tuberous Sclerosis Complex Consensus Group. (2013). Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 49(4):255-265.
- Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. (2016). Update on the Diagnosis and Management of Renal Angiomyolipoma. J Urol. 195(4 Pt 1):834-846.
- Kothary N, Soulen MC, Clark TW, Wein AJ, Shlansky-Goldberg RD, Crino PB, et al. (2005). Renal angiomyolipoma: long-term results after arterial embolization. J Vasc Interv Radiol. 16(1):45-50.
- Nozadze G, Larsen SB, Heerwagen S, Juhl Jensen R, Lönn L, Røder MA. (2021). Selective arterial embolization of renal angiomyolipomas: A 10-year experience. BJUI Compass. 3(1):86-92.
- Bardin F, Chevallier O, Bertaut A, Delorme E, Moulin M, Pottecher P, et al. (2017). Selective arterial embolization of symptomatic and asymptomatic renal angiomyolipomas: A retrospective study of safety, outcomes and tumor size reduction. Quant Imaging Med Surg. 7(4):383-390.
- Boorjian SA, Frank I, Inman B, Lohse CM, Cheville JC, Leibovich BC, et al. (2007). The role of partial nephrectomy for the management of sporadic renal angiomyolipoma. Urology. 70(6):1064-1068.
- Heidenreich A, Hegele A, Varga Z, von Knobloch R, Hofmann R. (2002). Nephron-sparing surgery for renal angiomyolipoma. Eur Urol. 41(3):267-273.
- Yip SK, Tan PH, Cheng WS, Li MK, Foo KT. (2000). Surgical management of angiomyolipoma: nephron-sparing surgery for symptomatic tumour. Scand J Urol Nephrol. 34(1):32-35.
- Fernández-Pello S, Hora M, Kuusk T, Tahbaz R, Dabestani S, Abu-Ghanem Y, et al. (2020). Management of Sporadic Renal Angiomyolipomas: A Systematic Review of Available Evidence to Guide Recommendations from the European Association of Urology Renal Cell Carcinoma Guidelines Panel. Eur Urol Oncol. 3(1):57-72.
- Bishay VL, Crino PB, Wein AJ, Malkowicz SB, Trerotola SO, Soulen MC, et al. (2010). Embolization of giant renal angiomyolipomas: technique and results. J Vasc Interv Radiol. 21(1):67-72.
- Patatas K, Robinson GJ, Ettles DF, Lakshminarayan R. (2013). Patterns of renal angiomyolipoma regression post embolisation on medium- to long-term follow-up. Br J Radiol. 86(1024):20120633.
- Inci O, Kaplan M, Yalcin O, Atakan IH, Kubat H. (2006). Renal angiomyolipoma with malignant transformation, simultaneous occurrence with malignity and other complex clinical situations. Int Urol Nephrol. 38(3-4):417-426.
- Faddegon S, So A. (2011). Treatment of angiomyolipoma at a tertiary care centre: the decision between surgery and angioembolization. Can Urol Assoc J. 5(6):E138-E141.
- Andersen PE, Thorlund MG, Wennevik GE, Pedersen RL, Lund L. (2015). Interventional treatment of renal angiomyolipoma: immediate results and clinical and radiological follow-up of 4.5 years. Acta Radiol Open. 4(7):2058460115592442.
.png)
.png)
.png)
.png)