Current Issue Volume 9, Issue 1 - 2025
Investigation into the Pharmacognostic and Antimicrobial Properties of the Leaf Extract of Bridelia Ferruginea Benth (Euphorbiaceae)
Anikwe Chidera Celestine1,2, Ezegbe Chekwube Andrew3,4,*, Ugwu Emmanuel Oforma1, Ogbozor Maduaburochi Christian1, Ozioko Diana Chinenyenwa1, Eneje Jude Chetachukwu1, Ugorji Anita Chidera4, Uchenna Chiamaka Precious4, Onyekwere Favour Ezinne4, Odo Kenechi Benjamin4, Ezugwu Christopher Obodike1
1Department of Pharmacognosy and Environmental Medicine, University of Nigeria, Nsukka, Enugu State, Nigeria
2Department of Clinical Pharmaceutical and Biological Sciences, University of Hertfordshire, England United Kingdom
3Human and Natural Science Center, ABC Federal University Santo Andre, Sao Paulo, Brazil
4Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nsukka, Enugu State, Nigeria
*Corresponding author: Ezegbe, Chekwube Andrew, Human and Natural Science Center, ABC Federal University Santo Andre, Sao Paulo, Brazil & Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nsukka, Enugu State, Nigeria, Tel: +2348038042802, E-mail: [email protected]
Received Date: November 10, 2024
Published Date: January 09, 2025
Citation: Celestine AC, et al. (2025). Investigation into the Pharmacognostic and Antimicrobial Properties of the Leaf Extract of Bridelia Ferruginea Benth (Euphorbiaceae). Mathews J Pharma Sci. 9(1):41.
Copyrights: Celestine AC, et al. © (2025).
ABSTRACT
Introduction: Bridelia ferruginea is a well-known shrub that is used in traditional medicine in the management of certain diseases such as dysentery, arthritis and constipation. Aim/objectives: to ascertain the validity of the use of B. ferruginea as an antimicrobial agent, to evaluate the pharmacogenetic profile and differentiate it from adulterated one, to detect the secondary metabolites present in the leaf extract and to compare the plants antimicrobial activity with standard antimicrobial agents. Methods: The leaves of Bridelia ferruginea were plucked from the stem and dried in the shade for approximately 1 week. The leaves were ground into moderately coarse powder using mortar and pestle. A 400 g quantity of the grounded leaves were packed into a glass jar and macerated with methanol for 24 hours. The extracts were then filtered, distilled and concentrated in a rotary evaporator at optimum temperature of 40.5 oC and was brought to complete dryness over a water bath. The flask containing the extract was allowed to cool and the extract was then collected. Results: The MIC values of the Bridelia ferruginea extract against susceptible organisms (S. aureus, B. subtilis and C. albicans) recorded results of 9.5 ± 0.21mm, 7.0 ± 0.15 mm and 9.0 ± 0.20 mm respectively without any significant difference (p < 0.05). The values of the inhibitory zone diameter obtained ranged from 7.0 ± 0.01mm to 9.5 ± 0.02 mm for C. albicans and S. aureus respectively, without any significant difference (p < 0.05). From the results obtained, the extract was more effective only against Gram positive bacteria. The phytochemical analysis of the leaves of Bridelia ferruginea showed the presence of secondary metabolites. Conclusion: The study indicates the enormous potential of B. ferruginea with anti-microbial properties. Due to its wide application, the phytochemicals present has been documented, and they could be useful in lead compound discovery.
Keywords: Bridelia ferruginea, Phytochemicals, Metabolites, Anti-microbials, Extract.
INTRODUCTION
Over the years, the knowledge and practice of traditional medicine has majorly influenced drug development [1]. Majority of the conventional medicines used all over the world originated from plants or its related compounds. Drug companies have leveraged on this advantage by the continuous development of herbs into conventional medicine [2]. A lot of disease management has now resorted to the use of medicinal plants and herbs especially in developing countries like Nigeria. The reason for such has been attributed to inaccessibility to conventional healthcare, cost and cultural acceptability [3].
Bridelia ferruginea is a well-known medicinal plant that belongs to the Euphorbiaceae family. Its pharmacological properties have been documented [4]. Some of the plant parts like fruits, leaves, barks and roots have been commonly used as decoctions, in arthritis management, dysentery, constipation, diarrhea, skin and intestinal infections [5]. According to Aja et al, 2013, [6] the antimicrobial, repellant and fibroblast growth stimulation properties of the leaves of B. ferruginea has been documented. The stem and stem bark have demonstrated anti-inflammatory, antioxidant, antimicrobial, antityphoid, anthelmintic and analgesic properties [7-15].
The antimicrobial activities of leaf extracts of B. ferruginea against Staphylococcus aureus, Shigella dysenteriae and Escherichia coli, and Bacillus subtilis has been reported by Fadeyi and Akpan [16]. Bridelia Ferruginea is a shrub that is commonly found in fields especially in the Northern region axis and some African countries like Congo, East Africa, Angola and Zimbabwe. There is limited information on the leave extract of B. ferruginea and its antimicrobial properties [17-20].
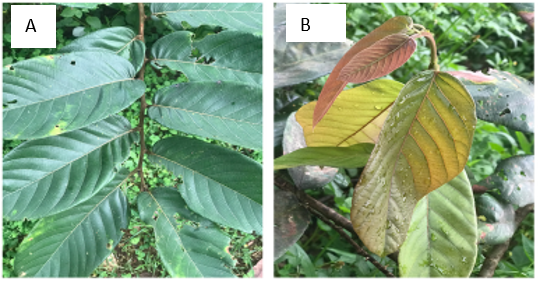
Figure 1. A) Matured leaves before reproduction, B) New leaves [20].
Aim and objectives of this work were to ascertain the validity of the use of B. ferruginea as an antimicrobial agent, to evaluate the pharmacogenetic profile and differentiate it from adulterated one, to detect the secondary metabolites present in the leaf extract and to compare the plants antimicrobial activity with standard antimicrobial agents.
MATERIALS AND METHOD
Materials
Bridelia ferruginea leaves were collected from Obukpa Nsukka in Enugu state, Dimethylsulphoxide(DMSO) (Sigma-Aldrich), Nutrient agar (Oxoid Ltd, England), sterile distilled water, cotton wool, indelible marker (UNN), Sabouraud Dextrose Agar, standard antibiotic (Gentamycin), Methanol 95%, chloroform, ammonia solution, ethanol,pPicric acid solution (Oxoid Ltd, England), lead acetate solution, Wagner’s reagent -Mayer’s reagent Dragendorff, ferric chloride solution, dilute hydrochloric acid solution (Oxoid Ltd, England), glacial acetic acid, dilute sulphuric acid, sodium carbonate solution, 10% aqueous aluminium chloride solution (Oxoid Ltd, England), Bromine water, Gram positive bacteria: Staphylococcus aureus Bacillus subtilis Gram negative bacteria: Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhi Fungi Yeast (Candida albicans), Mould (Aspergillus niger) (Gattefosse Saint-Priest Cedex, France). All other reagents and solvents used were of analytical grade.
Method
Sourcing of the leaves of B. ferruginea
Bridelia ferruginea leaves were collected from Obukpa Nsukka in Enugu state and identified by a taxonomist, Mr. Felix Nwafor, a Botanist, in the Pharmacognosy Herbarium, University of Nigeria Nsukka, Enugu State Nigeria. A herbarium Voucher Number of PCG/UNN/04980 Euphorbiaceae (L) was assigned.
Extraction procedure
The leaves of Bridelia ferruginea were plucked from the stem and dried in the shade for approximately 1 week. The leaves were ground into moderately coarse powder using mortar and pestle. A 400 g quantity of the grounded leaves were packed into a glass jar and macerated with methanol for 24 hours. The extractives were then filtered, distilled and concentrated in a rotary evaporator at optimum temperature of 40.5 oC and was brought to complete dryness over a water bath. The flask containing the extract was allowed to cool and the extract was then collected [21-23].
Antimicrobial evaluation of the methanolic extract of B. ferruginea
Sterilization of materials
The pipettes, test tubes and the agar plates packed into metal canisters were sterilized using hot air oven at 170 oC for 1 hour. The oven was put off and the metal canisters brought out and allowed to cool before the materials inside were brought out for use. The culture media were sterilized using autoclave at 121 oC for 15 minutes.
Preparation of test microorganisms
The test organism’s suspensions were prepared by marching the turbid suspension with a Marcfarland standard 0.5. Stock solutions of microorganisms were subcultured and incubated at 37 oC and 25 oC for bacteria and fungi respectively for 18-24 hours before use, in order to get pure isolates. Standard suspensions of each test microorganism were made by transferring a colony from the subculture into 5 ml of sterile distilled water and adjusting the volume to obtain a cell population of 1.0 ×106 cfu/ml which was the standard inoculums size used in all the tests [24].
Preparation of culture media
In the preparation of sabouraud dextrose medium for screening of plant extract using fungi, 0.05mg/ml of chloramphenicol was incorporated into the medium to preclude interference or growth of bacteria in the fungi medium. A 250 mg quantity of chloramphenicol was dissolved in 5 ml of ethanol, then 1 ml was withdrawn from the solution and then made up to 100 ml of the sabouraud dextrose medium using distilled water. A 65 g quantity of sabouraud dextrose powder leaf was suspended in 1 litre of distilled water containing 0.05mg/ml chloramphenicol and it was soaked for 5 minutes until all the powder has dissolved. The mixture was swirled, and sterilized by autoclaving at 121 oC for 15 minutes, cooled to 48- 50 oC and then poured into a petri dish [25].
Preparation of Nutrient agar medium for bacteria Formulation
Nutrient agar Peptone 5.0 g, Beef extract 3.0 g, Sodium chloride 8.0 g, Agar no 2 12.0 g pH 7.3 ±2.28g of Nutrient agar powder was dispersed in 1 litre of deionized water and the mixture was soaked for 10 minutes. It was swirled to mix properly and then sterilized for 15 minutes at 121 oC. It was cooled to 47 oC, poured into a plate and stored away from direct sunlight [26].
Preparation of stock solution of plant extract
The stock solution of methanolic extract of Bridelia ferruginea (100 mg) was prepared by weighing 200 mg of the substance and dissolving some in 2ml of dimethylsulfoxide (DMSO) and agitating to complete dissolution. This was used as the stock solution, serial dilutions of the extract were obtained: 100mg/ml, 50mg/ml, 25mg/ml, 12.5mg/ml and 6.25mg/ml [26].
Preliminary antimicrobial screening (sensitivity test)
Preliminary antimicrobial screening of the methanolic extract was done using the cup plate agar diffusion method. A 0.2 ml portion of each test organism was added to 20 ml of sterile molten agar (bacteria and fungi media) in seven different nutrient agar plates, mixed thoroughly and allowed to equilibrate [27]. The seeded agar plates were allowed to set for about 10 minutes. A sterile cork borer, 8 mm in diameter, was then used to form cups at five different points in each plate. Each plate was inoculated with a microorganism and labeled appropriately. Afterwards, two drops of the methanolic extract, dissolved in dimethyl sulfoxide were introduced into each cup using sterile Pasteur pipettes. The plates were then allowed to stand for 30 minutes at room temperature to allow for pre-diffusion and then incubated at 37 oC for 24 hours. After incubation, the diameters of the zones of inhibition were measured in millimeter for the sensitive organisms [28,29].
Determination of minimum inhibitory concentration (MIC)
The MICs of methanolic extracts of the plant were determined against susceptible organisms using agar diffusion method [30]. Agar plates were prepared (seeded) with organisms in two different plates; Graded concentrations of the extracts (100mg/ml, 50 mg/ml, 25 mg/ml, 12.5 mg/ml, 6.25 mg/ml) were prepared. Six wells were bored on seeded plates using a sterile cork borer of 8 mm diameter. Each well was marked and 1ml volumes of the different dilutions introduced into them using a sterile pipette. The test was performed for both extracts using each test organism and each in duplicate and the plates were incubated at 37 oC for 24 hours (for bacteria) and 25 oC for 48 hours (for fungi). The inhibitory zone diameters were recorded by calculating the mean of each set of replicate inhibition zone diameters. The minimum inhibitory concentrations (MICs) were determined by plotting a graph of inhibitory zone diameters (IZD) against the logarithm of the concentrations. The above procedure was also carried out to determine the MIC of Gentamycin and Clotrimazole against the sensitive bacteria and fungi respectively.
Phytochemical analysis
Phytochemical analysis for crude drug includes:
Test for Resins
A 0.3 g of the crude drug was extracted with 10 ml of 96 % ethanol. The alcoholic extract was then poured into 20 ml of distilled water in a beaker and observed [31].
(b) Colour test: Another 0.3 g quantity of the crude drug was extracted with chloroform and the extract was concentrated to dryness. The residue was concentrated to dryness. The residue was redissolved in 3 ml of acetone and 3 ml of concentrated hydrochloric acid was added. The mixture was heated in a water bath for 30 minutes and observed.
Test for Alkaloids
A 0.5 g quantity of the extract was warmed on a water bath with 2 ml of 1% Sulphuric acid for 2 minutes. It was filtered and the filtrate used for the following tests;
- Mayer’s test 2 drops of Mayer’s (Potassium mercuric iodide) solution was added to 5ml of the filtrate. A creamy of white buff precipitate indicates the presence of alkaloid [32].
- Dragendorff’s test: A 2 drops quantity of Dragendorff’s reagent (potassium bismuth iodide) solution was added to 5ml of filtrate. A deep brick-red precipitate indicates the presence an alkaloid [33].
Test for Tannins
A 1g quantity of the methanolic extract of the leaves was boiled with 20 ml of water, cooled and filtered. The filtrate was then used for the following tests:
- Ferric chloride test: A 1 ml quantity of the filtrate was diluted to 5 ml with water and a few drops of ferric chloride (0.1%) were added. A greenish brown precipitate indicates the presence of tannins.
- Bromine water test: A 1ml quantity of the filtrate was added 2 drops of bromine water. A pale brown precipitate indicates the presence of tannins [34].
Test for Flavonoids
A 0.2 g quantity of the methanolic extract was heated with 10 ml of ethyl acetate in a boiling water bath for 3 minutes. The mixture was filtered and the filtrate was used for the following tests;
- Ammonia test: A 4 ml quantity of the filtrate was shaken with 1ml of dilute ammonia. A yellow coloration in ammonia layer indicates the presence of flavonoids.
- Aluminium chloride test: A 4 ml quantity of filtrate was shaken with 1 ml of 10 % Aluminium chloride solution. A yellow collour in the aluminium layer indicates the presence of flavonoids [35].
Test for Saponins
A 1 g quantity of the methanolic extract of the leaves was boiled with 10 ml of distilled water for 10 minutes, filtered while hot and filtrates cooled and the following tests were performed;
- Frothing test
A 2.5 ml quantity of the filtrate was diluted to 10ml with water and shaken vigorously for 2minutes. Persistent foaming (stable froth) indicates the presence of saponins.
- Emulsion test
2 drops of olive oil was added to the filtrate and shaken vigorously. Formation of stable emulsion indicates the presence of saponins [36].
Test for Steroids
A 1g quantity of the methanolic extract was boiled under reflux with 18ml of ethanol and filtered. The filtrate was concentrated to about 15 ml and 10 ml of water was added to the concentrated filtrate and allowed to stand for about one hour and the waxy material filtered. The filtrate was extracted with chloroform and used for the following tests;
- Salkiwski test: A 1ml quantity of sulphuric acid was carefully added to 0.5 ml of the concentrated chloroform extract in a test tube, so as to form a lower layer and heated for 15 minutes. A red interphase between the 2 layers indicates the presence of steroids [35,36].
Test for Terpenoids
- Liebermann’s test: A 0.5 ml quantity of the concentrated chloroform extract in a test tube was evaporated to dryness. The residue was dissolved in a 0.5 ml glacial acetic acid and 1 ml sulphuric acid and heated for 15 minutes. A red magnate or violet blue indicates the presence of terpenoids [38].
Test for oils
A 0.1 g quantity of the powdered drug was pressed between filtered paper and the paper observed. Translucency of the filter paper indicates the presence of oil [39].
Test for Glycosides
A 5ml quantity of dilute sulphuric acid was added to 0.1 g of the powdered drug in a test tube and boiled for 15 minutes on a water bath, then cooled and neutralized with 20 % potassium hydroxide solution. A 10 ml quantity of a mixture of equal parts of Fehling’s solution 1 and 2 were added and boiled for 5 minutes. It was then observed for the presence of brick-red precipitate [40].
Test for reducing sugars
A 0.2g quanity of the powdered drug was shaken vigorously with 10 ml of distilled water and filtered. The filtrate was used in the following tests [41]:
- Fehling’s test: To 1 ml portion of the filtrate, equal volumes of fehling’s solution 1 and 11 were added and boiled on water bath for few minutes. A brick-red precipitate indicates the presence of reducing sugars.
- Benedicts’s test: To 1 ml portion of the filtrate was added 2 ml of Benedict’s reagent. A rusty brown precipitate indicates the presence of reducing sugar.
Test for Protein
A 0.5 g portion of the powdered drug was extracted with 10 ml of distilled water and the filtrate was used for the following tests:
- Million’s test: To a little portion of the filtrate in a test tube, two drops of Million’s reagent were added. A white precipitate indicates the presence of proteins.
- Xanthoproteic reaction test: A 5ml quantity of the filtrate was heated with few drops of concentrated nitric acid. A yellow colour which changes to orange on addition of alkali indicates the presence of proteins [41].
- Picric acid test: A few drops of picric acid were added to a little portion of the filtrate and observed.
Test for Carbohydrates
A 0.1 g quantity of the powdered material was boiled in 2ml of water and filtered. To the filtrate, few drops of Naphthol solution in ethanol (Molisch reagent) was added. Concentrated sulphuric acid was then gently poured down the side of the test tube to form a lower layer. A purple interfacial ring indicates the presence of carbohydrates [42].
Test for acidity
The plant material was placed in a clean dry test tube and sufficient water was added. This was warmed in hot water bath and then cooled. A piece of water-wetted litmus paper was dipped into the filtrate and colour change on the litmus paper was observed.
Microscopic evaluation
A little quantity of the powdered drug was placed on a glass slide and a drop of chloral hydrate was added. It was covered with a cover slip and passed three times over the flame from a Bunsen burner. It was then focused on the microscope and viewed.
Standardization procedures
Total Ash value
A tarred nickel crucible was ignited to a constant weight at a dull red heat, cooled and stored in a desiccator. A 2 g quantity of the powdered material was weighed into the nickel crucible and heated gently until all the moisture had been completely removed, after which the material was heated further until residue was free from carbon. The residue was cooled in a desiccator and weighed. The heating and cooling continued until a constant weight was achieved and the total ash value calculated from the results [43-45].
Acid Insoluble Ash Value
The total ash obtained in total ash value experiment was transferred to a beaker containing 25 ml of dilute hydrochloric acid and heated to boiling on a water bath for five minutes. It was then filtered through ash-less filter paper and the beaker and crucible were washed with distilled water until free from acid. The filter paper was dried in the oven, folded into a narrow cone and inserted into a tarred nickel crucible and heated until it was completely ashed. The residual ash was then heated more strongly and after cooling in a desiccator, it was weighed [43-45].
Sulphated Ash value
A nickel crucible was ignited to a constant weight at a dull red heat. A 2g quantity of the material was spread over the bottom of the crucible and was reweighed. The material was moistened with dilute sulphuric acid and ignited at low heat initially to burn off the carbon content. The crucible was cooled in a desiccator and more dilute sulphuric acid was added and heating continued with occasional cooling until a constant weight was obtained [46].
Moisture content
A tarred evaporating dish was heated to a constant weight and stored in a desiccator. A 2g quantity of the powdered material was added and the dish was kept in an oven maintained at a temperature of 105 oC and was allowed to dry until a constant weight was obtained.
Data analysis
All the measurements were repeated at least thrice and the data obtained analyzed by Student t-test and One-Way Analysis of Variance (ANOVA). Statistical analysis was performed using Statistical Product and Services Solution software (SPSS, version 22.0 Inc., Chicago IL, USA) and Excel Microsoft Office version 2012. The results were presented as mean ± SD, and statistical differences between means considered significant at (p < 0.05).
RESULTS AND DISCUSSION
Antimicrobial evaluation of Bridelia ferruginea methanolic leaf extract
After the incubation period for the bacteria and fungi at 37 oC for 24 hours and 25 oC for 48 hours respectively, the following antimicrobial result for the methanolic extract and standard antimicrobial agents (Gentamycin and Clotrimazole) were obtained as shown in Table 1 below. The MIC values of the Bridelia ferruginea extract against susceptible organisms are shown in Table 1. S. aureus, B. subtilis and C. albicans recorded results of 9.5 ± 0.21 mm, 7.0 ± 0.15mm and 9.0 ± 0.20 mm respectively without any significant difference (p < 0.05).
Table 1. Sensitivity of the various test organisms to the extracts of Bridelia ferruginea
|
Test organism |
Result obtained (mean ± SD) |
|
Escherichia coli |
++ |
|
Staphylococcus aureus |
9.5 ± 0.21 |
|
Salmonella typhi |
++ |
|
Pseudomonas aeruginosa |
++ |
|
Klebsiella pneumonia |
++ |
|
Bacillus subtilis |
7.0 ± 0.15 |
|
Candida albicans |
9.0 ± 0.20 |
|
Aspergillus niger |
++ |
The inhibitory zone diameter of the test organisms is represented in Table 2. The values obtained ranged from 7.0 ± 0.01 to 9.5 ± 0.02 for C. albicans and S. aureus respectively at 100 mm, without any significant difference (p < 0.05). According to Irobi et al; the inhibitory zone diameter at 4-30mm diameter, showed that the results obtained ranged from +2 to +3 for the test organisms [47,48].
Table 2. Inhibitory zone diameters of sensitive microorganisms using different concentrations of the extract
|
Test organism |
Average zone of inhibition (mm, mean ± SD) |
||||
|
100 |
50 |
25 |
12.5 |
6.25 |
|
|
Staphylococcus aureus |
9.5 ± 0.02 |
6.0 ± 0.02 |
5.3 ± 0.01 |
2.0 ± 0.01 |
1.8 ± 0.01 |
|
Bacillus subtilis |
9.0 ± 0.02 |
7.5 ± 0.02 |
5.0 ± 0.01 |
2.0 ± 0.01 |
0.8 ± 0.01 |
|
Candida albicans |
7.0 ± 0.01 |
4.3 ± 0.01 |
3.5 ±0.01 |
2.5 ± 0.01 |
- |
Table 3. Spectrum of activity of leaf methanolic extract against bacteria and fungi
|
Microorganism |
Concentration of extract (mg/ml) |
IZD = L (mm) |
L2 (mm) |
|
S aureus |
100.00 ± 0.23 |
9.50 ± 0.20 |
90.25 ± 0.23 |
|
50.00 ± 0.21 |
7.50 ± 0.10 |
56.25 ± 0.20 |
|
|
25.00 ± 0.10 |
5.00 ± 0.10 |
25.00 ± 0.10 |
|
|
12.50 ± 0.01 |
2.00 ± 0.01 |
4.00 ± 0.10 |
|
|
6.25 ± 0.01 |
0.80 ± 0.01 |
-------- |
|
|
B. subtilis |
100 ± 0.23 |
9.00 ± 0.03 |
0.64 ± 0.03 |
|
|
50 ± 0.10 |
7.00 ± 0.01 |
81 ± 0.23 |
|
|
25 ± 0.10 |
3.20 ± 0.01 |
49 ± 0.21 |
|
|
12.50 ± 0.01 |
------- |
10.24 ± 0.01 |
|
|
6.25 ± 0.01 |
-------- |
--------- |
|
C. albicans |
100 ± 0.23 |
7.00 ± 0.01 |
49 ± 0.10 |
|
50 ± 0.10 |
4.30 ± 0.01 |
18.49 ± 0.10 |
|
|
25 ± 0.10 |
3.50 ± 0.01 |
12.25 ± 0.01 |
|
|
12.50 ± 0.01 |
2.50 ± 0.01 |
6.25 ± 0.01 |
|
|
6.25 ± 0.01 |
----- |
------- |
Table 4. Spectrum of activity of the standard antibiotic Gentamycin against bacteria (Inhibition zone diameter IZD, mean ± SD)
|
Microorganism |
Concentration of extract (mg/ml) |
IZD = L (mm) |
L2 (mm) |
|
S aureus |
100.00 ± 0.23 |
41.00 ± 0.20 |
1681 ± 0.20 |
|
50.00 ± 0.21 |
32.60 ± 0.10 |
1062 ± 0.21 |
|
|
25.00 ± 0.10 |
25.60 ± 0.10 |
655 ± 0.20 |
|
|
12.50 ± 0.01 |
20.00 ± 0.01 |
400 ± 0.10 |
|
|
6.25 ± 0.01 |
9.00 ± 0.01 |
81 ± 0.10 |
|
|
B. subtilis |
100.00 ± 0.23 |
35.00 ± 0.03 |
1225 ± 0.03 |
|
|
50.00 ± 0.10 |
30.00 ± 0.01 |
900 ± 0.23 |
|
|
25.00 ± 0.10 |
20.00 ± 0.01 |
400 ± 0.21 |
|
|
12.50 ± 0.01 |
14.00 ± 0.10 |
196 ± 0.01 |
|
|
6.25 ± 0.01 |
8.00 ± 0.01 |
--------- |
Table 5. Activity of the standard antifungal agent Clotrimazole against the Fungi (IZDs)
|
Test organism |
Concentration of clotrimazole (mg/ml) |
IZD (mm) |
IZD2 (mm) |
|
C. albicans |
100.00 ± 0.20 |
28.00 ± 0.02 |
784.00 ± 0.20 |
|
|
50.00 ± 0.10 |
21.00 ± 0.01 |
441.00 ± 0.20 |
|
|
25.00 ± 0.01 |
15.00 ± 0.01 |
225.00 ± 0.02 |
|
|
12.50 ± 0.01 |
9.00 ± 0.02 |
81.00 ± 0.01 |
|
|
6.25 ± 0.01 |
6.00 ± 0.01 |
36 ± 0.01 |
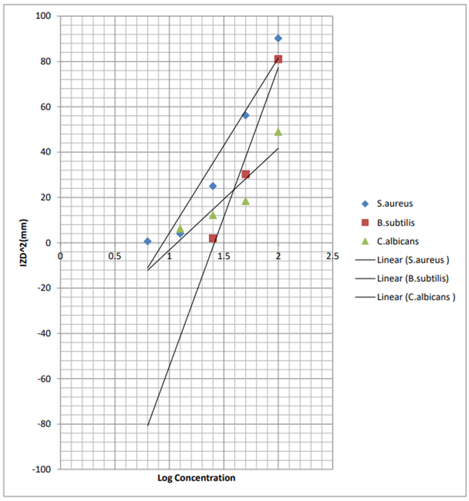
Figure 1. A graph of IZD2 against concentrated methanolic extracts of Bridelia ferruginea for susceptible organisms.
Figure 2. A graph of IZD2 against log concentration of standard antibiotics (Gentamycin and clotrimazole).
The antimicrobial activity was more pronounced against bacteria than fungi. The MIC illustrates a decreasing inhibitory effect of the leaf extracts as the concentration decreases. This implies that antimicrobial activity of a substance is concentration-dependent and a function of the active ingredient reaching an organism [49]. From the results, the extract seems to be effective only against Gram positive bacteria and not Gram-negative bacteria. The reason for the different sensitivity between Gram positive and Gram-negative bacteria could be ascribed to the morphological differences between these microorganisms. Gram negative bacteria have an outer phospholipidic membrane carrying the structural lipopolysaccharide components, which makes the cell wall partially impermeable. The Gram-positive bacteria are thus, more susceptible, having only an outer peptidoglycan layer which is not an effective permeability barrier. From the minimum inhibitory concentration, it revealed that it has greatest activity on Staphylococcus aureus (MIC = 8.91), followed by Candida albicans (MIC = 12.02mg/ml) and then, B. subtilis (MIC = 25.12) on which it has the least effect. On comparing the methanolic extract of the Bridelia ferruginea with the standard drugs (Gentamycin and Clotrimazole), it is clear that the standard drugs have greater activity [50].
Table 6. MIC determination from the graph for the standard antibiotics (Gentamycin and clotrimazole)
|
Test organism |
Intercept on log conc axis |
Antilog of intercept (MIC) mg/ml |
|
S. aureus |
0.80 |
6.31 |
|
B. subtilis |
0.85 |
7.08 |
|
C. albicans |
0.90 |
7.94 |
Previous studies demonstrated the presence of flavonoid components. Other authors showed the presence of terpenes and isomers of nepetalactone in Nepeta cataria and their bacteriostatic and fungistatic properties [51]. However, it is difficult to compare the data of this study with literature because several variables influence the results, such as the type of plant used, the climatic and environmental factors of the plant, the method of extraction and the use of appropriate solvents. The presence of these chemical constituents underscores the importance of these plants in medicine. According to Prasathkumar et al, [52], all alkaloids contain quinine, morphine or reserpine which are used for malaria, pain relief and valuable tranquilizers respectively. Alkaloids are one of the largest groups of phytochemicals in plants, having amazing effects on humans and has led to the development of powerful pain killer medications. This suggests that B. ferruginea can be a useful source for malaria drug, pain relief and tranquilizer. The leaf extract has been reported to reduce plasma glucose levels in diabetic patients [53]. However, further experimental and research efforts on the plants and their extracts are needed to be able to specify the pharmacological implication.
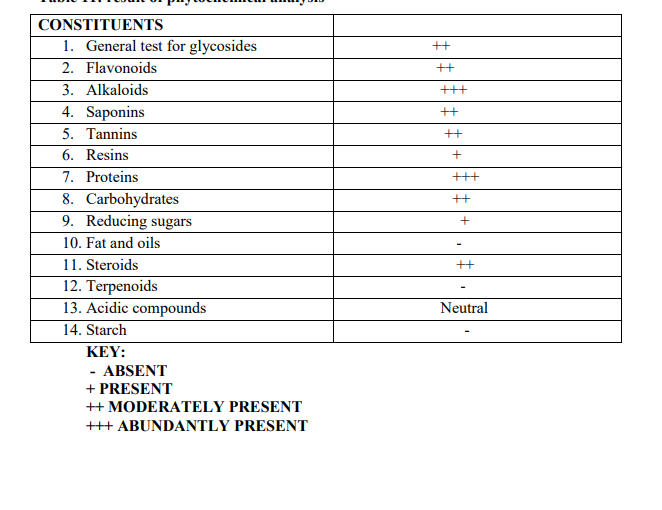
Table 7. Phytochemical analysis
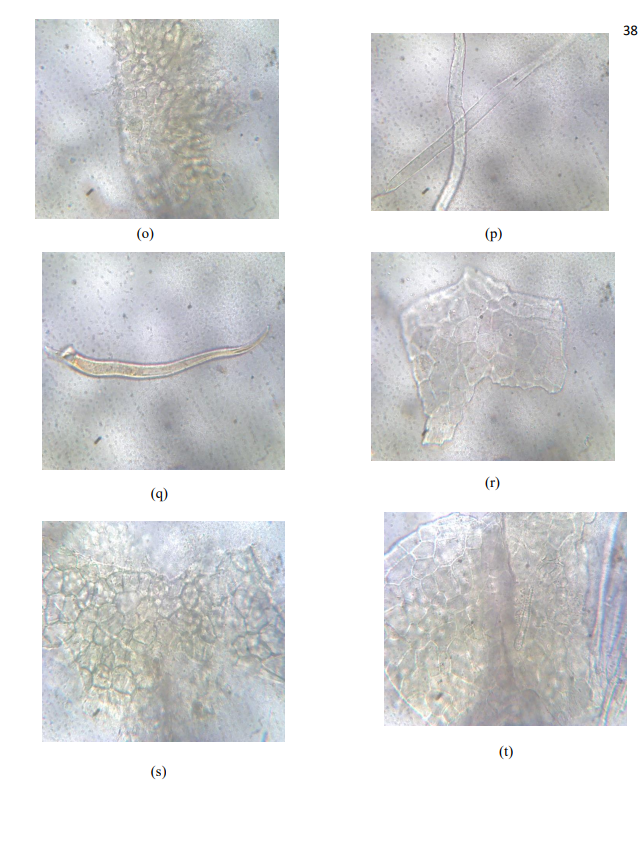
The microscopic evaluation showed the presence of prism calcium oxalate, bundle of fibers, circular palisade cells, trichomes, annular xylem vessels, epidermal cells with no stomata, large phloem fibers. The standardization of crude drugs has become very necessary and important because they are used in quality control especially to determine the quality, purity, strength and if adulterated, the nature of the adulterants. It has also been used to determine the percentage yield of the crude Bridelia ferruginea material as indicated in the work and also in solving the problem of spoilage of herbal drugs.
- Bundle of annular xylem vessels and group of fibres
- Circular palisade cells
- Clothing unicellular trichome and a prism calcium oxalate
- Elongated clothing multicellular trichome
- Elongated non glandular trichome
- Elongated non glandular trichome
- Fractured multicellular trichome
- Group of fibres
- Large annular xylem vessels
- Large phloem fibres
- Large upper epidermal cells with no trichome
- Lower epidermis showing anomocytic type of stomata
- Multicellular elongated trichome
- Multicellular trichome
- Numerous palisade cells
- Pair of elongated unicellular non glandular trichome
- Unicellular clothing trichome
- Upper epidermis showing cicatrix
- Upper epidermis showing palisade cells with no stomata
- Upper epidermis with attached trichome
The result of the standardization test is presented in Table 8. The moisture content of the leave extract was 1.05 ± 0.02, the total ash value was 5.00 ± 0.01, the sulphated ash and acid insoluble ash ranged from 2.00 ± 0.01 to 1.15 ± 0.01 respectively.
Table 8. Standardization
|
Parameter |
Result |
|
Moisture content |
1.05 ± 0.02 |
|
Total ash |
5.00 ± 0.01 |
|
Acid insoluble ash |
1.15 ±0.01 |
|
Sulphated ash |
2.00 ± 0.01 |
Pharmacognostic studies performed on the leaves support most of the claims with a few traditional uses yet to be approved. The study indicates the enormous potential of B. ferruginea with anti-microbial properties. Due to its wide application, the phytochemicals present has been documented, and they could be useful in lead compound discovery. A lot of untapped potentials for further investigation still remains despite its wide application.
ACKNOWLEDGEMENTS
The authors wish to thank all the Lecturers and laboratory staffs in the Department of Pharmacognosy and Environmental Medicine, UNN for their support and assistance during the research.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHORS CONTRIBUTIONS
Anikwe Celestine Chidera: Conceptualization, Supervision, Ezegbe Chekwube Andrew: Methodology, Writing, Ugwu Emmanuel Oforma: Methodology, Writing
Ogbozor Maduaburochi Christian: Methodology, Writing, Ozioko Diana Chinenyenwa: Methodology, Writing, Eneje Jude Chetachukwu: Methodology, Writing, Ugorji Anita Chidera: Methodology, Writing, Uchenna Chiamaka Precious: Methodology, writing, Ezugwu Christopher Obodike: Supervision, Conceptualization
REFERENCES
- Adebayo EA, Ishola OR. (2009). Phytochemical and antimicrobial screening of the crude extracts from the root, stem bark and leaves of Bridelia ferruginea. Afr J Biotechnol. 8(4):650-653.
- Adebayo OA, Joshua OB. (2018). Evaluation of anthelmintic potential of aqueous extract of the stem bark of Bridelia ferruginea (benth) euphorbiaceae. J Pharmaceut Res Int. 24(6):1-6.
- Adetutu A, Morgan WA, Corcoran O. (2011). Antibacterial, antioxidant and fibroblast growth stimulation activity of crude extracts of Bridelia ferruginea leaf, a wound healing plant of Nigeria. J Ethnopharmacol. 133(1):116-119.
- Afolabi OB, Oloyede OI, Agunbiade SO. (2018). Inhibitory potentials of phenolic-rich extracts from Bridelia ferruginea on two key carbohydrate-metabolizing enzymes and Fe2þ-induced pancreatic oxidative stress. J Integr Med. 16(3):192-198.
- Afolayan M, Srivedavyasasri R, Asekun OT, Familoni OB, Ross SA. (2019). Chemical and biological studies on Bridelia ferruginea grown in Nigeria. Nat. Prod. Res. 33 (2), 287–291. Aja, P., 2013. Evaluation of anti-diabetic and liver enzymes activity of aqueous extracts of moringa oleifera and Bridelia ferruginea leaves in alloxan induced diabetic albino rats. Int J Biochem Res Rev. 3(3):248-258.
- Aja P. (2013). Evaluation of anti-diabetic and liver enzymes activity of aqueous extracts of moringa oleifera and Bridelia ferruginea leaves in alloxan induced diabetic albino rats. Int J Biochem Res Rev. 3(3):248-258.
- Akinpelu DA, Olorunmola FO. (2000). Antimicrobial activity of Bridelia ferruginea fruit. Fitoterapia. 71(1):75-76.
- Akinsete TO, Adebayo-tayo BC. (2017). The phytochemical and antimicrobial potentials of the crude extracts of Bridelia ferruginea and the extracellular biosynthesized silver nanoparticles. JAMPS. 14(3):1-13.
- Akubue PI, Mittal GC. (1982). Clinical evaluation of a traditional herbal practice in Nigeria: a preliminary report. J Ethnopharmacol. 6(3):355-359.
- Abubakar MS, Musa AM, Ahmed A, Hussaini IM. (2007). The perception and practice of traditional medicine in the treatment of cancers and inflammations by the Hausa and Fulani tribes of Northern Nigeria. J Ethnopharmacol. 111(3):625-629.
- Alowanou GG, Olounlade PA, Akouedegni GC, Faihun AML, Koudande DO. (2019). In vitro anthelmintic effects of Bridelia ferruginea, Combretum glutinosum, and Mitragyna inermis leaf extracts on Haemonchus contortus, an abomasal nematode of small ruminants. Parasitol Res. 118(4):1215-1223.
- Ampofo O. (1979). The Practice of Phytotherapy in Ghana. In: Sofowora EA, (Ed.). African Medicinal Plants. University of Ife Press, Ile-Ife. p. 67.
- Awodele O, Amagon KI, Agbo J, Prasad MNV. (2015). Toxicological evaluation of the aqueous stem bark extract of Bridelia ferruginea (Euphorbiaceae) in rodents. Interdiscipl Toxicol. 8(2):89.
- Ayensu ES. (1978). Medicinal Plants of West Africa. Reference Publications, Inc., Algonac, Michigan. p. 162.
- Aziato L, Antwi HO. (2016). Facilitators and barriers of herbal medicine use in Accra, Ghana: an inductive exploratory study. BMC Compl Alternative Med. 16(1):1-9.
- Fabiyi OA, Atolani O, Adeyemi OS, Olatunji GA. (2012). Antioxidant and cytotoxicity of Bridelia ferruginea leaves acetate fraction from. Asian Pac J Trop Biomed. 2(2):S981-S984.
- Gill LS. (1992). Ethnomedicinal Uses of Plants in Nigeria. University of Benin Press, Benin, Nigeria. p. 276.
- Hertog MGL, Feskens EJM, Kromhout D, Hertog MGL, Hollman PCH, Hertog MGL, et al. (1993). Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 342(8878):1007-1011.
- Houndjo CF, Agbodjogbe W, Assogba FM, Kohoude JM, Ayedoun MA, Dansou PH, et al. (2017). Comparative study of Antihyperglycemic activity of aqueous extracts from the leaves of Bridelia ferruginea, Lophira lanceolata and Oxytenanthera abyssinica, with their mixture. Int J Curr Res Chem Pharmaceut Sci. 4(11):22-33.
- Ikechukwu AA, Ibiam UA, Okechukwu PCU, Inya-Agha OR, Obasi UO, Chukwu DO. (2015). Phytochemistry and acute toxicity study of Bridelia ferruginea extracts. World J Med Sci. 12(4):397-402.
- Irobi ON, Moo-Young M, Anderson WA, Daramola SO. (1994). Antimicrobial activity of bark extracts of Bridelia ferruginea (Euphorbiaceae). J Ethnopharmacol. 43(3):185-190.
- Jose RA, Kayode J. (2009). The Effect of Bridelia ferruginea Bark extracts on some pathogenic micro-organisms. Ethnobotanical Leaflets. 2009(8):8.
- Kareem KT, Kareem SO, Adeyemo OJ, Egberongbe RK. (2010). In vitro antimicrobial properties of Bridelia ferruginea on some clinical isolates. Agric Biol J N Am. 1(3):416-420.
- Kolawole OM, Oladoyinbo SO, Agbede OO, Adu FD. (2006). The effect of Bridellia ferruginea and Senna alata on plasma glucose concentration in normoglycemic and glucose induced hyperglycemic rats. Ethnobotanical Leaflets. 10:209-218.
- Kolawole OM, Olayemi AB. (2003). Studies on the efficacy of Bridelia ferruginea benth extract for water purification. Niger J Pure Appl Sci. 18:1387-1394.
- Kolawole OM, Olayode JA, Oyewo OO, Kolawole CF. (2009). Toxicological renal effects of Bridelia ferruginea-treated wastewater in rats. Afr J Microbiol Res. 3(3):82-87.
- Lagnika L, Fantodji MH, Sanni A. (2012). Phytochemical study and antibacterial, antifungal and antioxidant properties of Bridelia ferruginea and Pteleopsis suberosa. Int J Pharmaceut Sci Res. 3(7):2130-2136.
- Loko LY, Alagbe O, Dannon EA, Datinon B, Orobiyi A, Thomas-odjo A, et al. (2017). Repellent Effect and Insecticidal Activities of Bridelia Ferruginea, Blighia Sapida, and Khaya Senegalensis Leaves Powders and Extracts against Dinoderus Porcellus in Infested Dried Yam Chips. Psyche. 2017: 5468202.
- MacRae WD, Towers GHN. (1984). Biological activities of lignans. Phytochemistry. 23(6):1207-1220.
- Magassouba FB, Diallo A, Kouyate M, Mara F, Mara O, Bangoura O, et al. (2007). Ethnobotanical survey and antibacterial activity of some plants used in Guinean traditional medicine. J Ethnopharmacol. 114(1):44-53.
- Maqbool M, Dar MA, Gani I, Mir SA, Khan M. (2019). Herbal medicines as an alternative source of therapy: a review. World J Pharm Pharmaceut Sci. 3(2):374-380.
- Mshana NR. (2000). Traditional Medicine and Pharmacopoeia: Contribution to the Revision of Ethnobotanical and Floristic Studies in Ghana. Organization of African Unity/Scientific, Technical & Research Commission.
- Ndukwe IG, Amupitan JO, Isah Y, Adegoke KS. (2007). Phytochemical and antimicrobial screening of the crude extracts from the root, stem bark and leaves of Vitellaria paradoxa (GAERTN. F). Afr J Biotechnol 6(16):1905-1909.
- Nene-Bi SA, Zahoui OS, Soro TY, Traore F. (2012). Diuretic and natriuretic activity of an aqueous extract of Bridelia Ferruginea Benth.(Euphorbiaceae) in normal rats. J Physiol Pharmacol Adv. 2(10):349-353.
- Nene-Bi SA, Ramachandran V, Vengal RP, Gopalakrishnan R, Dhanabal SP, Traore F. (2016). Subchronic toxicity studies of the aqueous stem bark extract of Bridelia ferruginea in Wistar rats. Bull Env Pharmacol Life Sci. 5(10):14-21.
- Nene Bi S, Traore F, Soro T, Souza A. (2009). Etudes phytochimique et pharmacologique de Bridelia ferruginea benth (euphorbiaceae) sur la motricite du Taenia coli de cobaye. Afr Sci Rev Int Sci Technol. 5(2):305-320.
- Ngueyem TA, Brusotti G, Caccialanza G, Finzi PV. (2009). The genus Bridelia: a phytochemical and ethnopharmacological review. J Ethnopharmacol. 124(3):339-349.
- Njamen D, Nkeh-chungag BN, Tsala E, Fomum ZT, Mbanya JC, Ngufor GF. (2012). Effect of Bridelia ferruginea (euphorbiaceae) leaf extract on sucrose-induced glucose intolerance in rats. Trop J Pharmaceut Res. 11(5):759-765.
- Odugbemi TO, Akinsulire OR, Aibinu IE, Fabeku PO. (2007). Medicinal plants useful for malaria therapy in okeigbo, ondo state, southwest Nigeria. Afr J Tradit Complementary Altern Med. 4(2):191-198.
- Olajide OA, Makinde JM, Awe SO. (1999). Effects of the aqueous extract of Bridelia ferruginea stem bark on carrageenan-induced oedema and granuloma tissue formation in rats and mice. J Ethnopharmacol. 66(1):113-117.
- Olajide OA, Makinde JM, Okpako DT, Awe SO. (2000). Studies on the anti-inflammatory and related pharmacological properties of the aqueous extract of Bridelia ferruginea stem bark. J Ethnopharmacol. 71(1-2):153-160.
- Olajide OA, Okpako DT, Makinde JM. (2003). Anti-inflammatory properties of Bridelia ferruginea stem bark Inhibition of lipopolysaccaride-induced septic shock and vascular permeability. J Ethnopharmacol. 88(2-3):221-224.
- Oliver-Bever B. (1986). Medicinal Plants in Tropical West Africa. UK: Cambridge University Press.
- Oloyede O, Ekiti A, Ojo OA, Onikanni S, Basiru A. (2014). In-vitro antioxidant activities of the stem bark extract fractions of Bridelia ferruginea. J Biol Agric Healthc. 4(3):1-7.
- Oloyede OI, Babalola SO. (2012). Invitro antioxidant activity of ethanolic extract of bridelia ferruginea (stem bark). Acad Res Int. 2(3):246-251.
- Onyenibe NS, Udogadi NS. (2019). Evaluation of antidiabetic role of Bridelia ferruginea methanol leaf extract in streptozocin induced diabetic male wistar rats. Pharm Pharmacol Int J. 7(6):264-269.
- Orabueze IC, Amudalat AA, Usman AA. (2016). Antimicrobial value of Olax subscorpioidea and Bridelia ferruginea on micro-organism isolates of dental infection. J. Pharmacogn. Phytochem. 5(5):398-406.
- Orafidiya LO, Lamikanra A, Adediji JA. (1990). Coagulation of milk as an index of astringency of the bark extract of Bridelia ferruginea benth and lime juice for the formulation of a traditional gargle ‘Ogun Efu. Phytother Res. 4(5):189-194.
- Owoade AO, Adetutu A, Airaodion AI, Ogundipe OO. (2018). Toxicological assessment of the methanolic leaf extract of Bridelia ferrugelia. J phytopharm. 7(5):419-424.
- Owoseni AA, Ayanbamiji TA, Ajayi YO, Ewegbenro IB. (2010). Antimicrobial and phytochemical analysis of leaves and bark extracts from Bridelia ferruginea. Afr J Biotechnol. 9(7):1031-1036.
- Pettit GR, Searcy JD, Tan R, Cragg GM, Melody N, Knight JC, et al. (2016). Antineoplastic agents. 585. Isolation of Bridelia ferruginea anticancer podophyllotoxins and synthesis of 4 - aza-podophyllotoxin structural modifications 1. J Nat Prod. 79(3):507-518.
- Prasathkumar M, Anisha S, Dhrisya C, Becky R, Sadhasivam S. (2021). Phytomedicine Plus Therapeutic and pharmacological efficacy of selective Indian medicinal plants – a review. Phytomedicine. 1(2):100029.
- Wabo PJ, Ngankam NJD, Bilong BCF, Mpoame M. (2011). A comparative study of the ovicidal and larvicidal activities of aqueous and ethanolic extracts of pawpaw seeds Carica papaya (Caricaceae) on Heligmosomoides bakeri. Asian Pac J Trop Med. 4(6):447-450.
.png)
.png)