Previous Issues Volume 6, Issue 3 - 2023
Inhibition of Plant Pathogens by Parthenium hysterophorus: An Investigation into Antimicrobial Properties
Mohd Dilshad1, Charu Gupta2,*
1Amity Institute of Organic Agriculture, Amity University Uttar Pradesh, India
2Amity Institute of Herbal Research & Studies, Amity University Uttar Pradesh, India
*Corresponding author: Charu Gupta, Amity Institute of Herbal Research & Studies, Amity University Uttar Pradesh, Sec-125, Noida-201313, UP, India; E-mail: [email protected].
Received Date: December 13, 2023
Published Date: December 30, 2023
Citation: Dilshad M, et al. (2023). Inhibition of Plant Pathogens by Parthenium hysterophorus: An Investigation into Antimicrobial Properties. Mathews J Nutr Diet. 6(3):28.
Copyrights: Dilshad M, et al. © (2023).
ABSTRACT
The goal of the present study was to assess the antimicrobial properties of leaf extracts of Parthenium hysterophorus using the agar well diffusion method against some plant pathogens. In the current investigation, the results illustrate that the leaf extracts of Parthenium hysterophorus exhibit a wide range of antimicrobial activity and can potentially be used as an alternative to chemical pesticides. This is the first complete study to report on antimicrobial effects of Parthenium hysterophorus against some selected plant pathogens with a view of searching a novel extracts to use as an alternative to chemical pesticides for sustainable agriculture production.
Keywords: Parthenium hysterophorus, Antimicrobial, Leaf Extract.
INTRODUCTION
The problem of invasive weeds has become a notable concern in recent years due to the ecological and socio-economic issues them pose [1]. Parthenium hysterophorus is a type of invasive weed from the Asteraceae family. This relatively short-lived, upright plant is known for its rapid growth and is particularly abundant in hot climates [2].
Commonly referred to as congress weed or gajar ghans (carrot weed) in India, it also goes by names like white top or feverfew (in the Caribbean) and ragweed parthenium (in the USA) [3-5]. It has gained recognition as the seventh most harmful weed globally, posing a significant threat to biodiversity.
Originally native to regions around the Gulf of Mexico, Southern North America, West Indies, and central South America, this weed has now spread to over 20 countries worldwide, spanning five continents and multiple islands [1]. Introduced to India as an ornamental plant in 1910, it initially failed to establish itself. However, in the 1950s, it reappeared in India and Australia due to contamination of wheat and pasture seeds imported from the United States. Its growth is promoted by nitrogen-rich waste from humans and livestock, explaining its proliferation near urban areas and settlements [6]. Despite being a weed causing allergies in humans, Parthenium hysterophorus L. has shown various potential medicinal applications [6]. Ramos et al. [7] noted its uses in treating neurological disorders, fever, urinary infections, dysentery, malaria, and as an emmenagogue. It has also shown promise as a remedy for hepatic amoebiasis [7].
Parthenium hysterophorus is a notable source of terpenoids, volatile oils, flavonoids, amino acids, sugars, and phenolic compounds. Parthenolide, the primary sesquiterpene lactone, along with parthenin and different solvent extracts, have demonstrated significant analgesic, anti-inflammatory, and antipyretic properties. These properties have led to its use in treating migraine headaches and fever. The application of crushed P. hysterophorus leaves externally has shown wound healing potential [7].
The plant exhibits strong resistance to plant-pathogenic microorganisms, possibly due to the presence of antimicrobial substances in its various parts. This study aimed to confirm the antimicrobial properties of Parthenium hysterophorus against specific plant pathogens, with the goal of finding an alternative extract for sustainable agricultural pest control, reducing reliance on chemical pesticides.
METHODS AND MATERIALS
The antimicrobial effectiveness was evaluated through a growth inhibition zone test utilizing the agar well diffusion method subsequent to the creation of the extracts.
Sample preparation
Roughly 500 grams of indigenous Parthenium hysterophorus plant leaves were gathered and subjected to oven drying for approximately 4 days at a temperature of 40-42°C, until a consistent weight was achieved. Subsequently, the dried leaves were pulverized into a fine powder. The resultant sample was stored at room temperature for subsequent utilization [8].
Preparation of Extracts
The extraction process was conducted employing the maceration technique. In this approach, 5 grams of leaf powder were combined with 50 ml each of ethyl acetate, methanol, ethanol, and 50% aqueous ethanol, separately. These mixtures were allowed to stand for duration of 3 days. Subsequent to this period, the resulting extracts were filtered and subjected to concentration on a water bath. The concentrated extract was then dissolved in Dimethyl Sulfoxide (DMSO) at a concentration of 1g/ml and preserved at a temperature of 4°C for subsequent utilization [9].
Bacterial and fungal strains
For the study, five distinct bacterial strains were chosen, encompassing 3 Gram-positive and 2 Gram-negative strains, primarily focusing on plant pathogens. The selected Gram-positive bacteria comprised Staphylococcus epidermidis, Bacillus subtilis (NCIM 2920), and Staphylococcus aureus (NCIM-5345), while the Gram-negative bacteria included Escherichia coli (NCIM 5346) and Pseudomonas aeruginosa. Additionally, a yeast strain, Candida albicans, was included. The fungal species utilized in this investigation consisted of Fusarium oxysporum (NCIM 1008), Aspergillus flavus (NCIM 1316), Penicillium citrinum (NCIM 1435), and Rhizopus stolonifera (NCIM 1139).
To facilitate the study, standard cultures of these bacteria and fungi were maintained at Amity AIHRS, Amity University UP, and Noida, India. Viability tests for each of the isolates were performed by reviving the organisms in nutrient agar medium and Sabouraud’s dextrose agar (SDA) medium, respectively. The bacterial stocks on nutrient agar medium (provided by Hi Media, Mumbai, India) were incubated at 37 °C for 24 hours, while the fungal stock was incubated at 28 °C for 3 days. Following incubation, the cultures were stored at 4 °C until they were ready to be employed for sensitivity testing.
Antibacterial activity testing using agar well diffused technique
The antibacterial efficacy of the leaf extracts from P. hysterophorus was evaluated using the agar well-diffusion technique. Initially, a pure culture of each bacterial strain was sub-cultured in nutrient broth and incubated at 37°C for 24 hours. A standardized inoculum of (100μL, 106 CFU/mL; 0.5 Mac-Farland), was evenly spread using a sterile spreader onto sterile agar plates to achieve a uniform growth across the plate surface. Once the plates were air-dried, sterile cork borers with a diameter of 6.0 mm were employed to create wells in the agar medium.
Following this, 50 μL of each extract was introduced into the wells of the agar plates. The plates were allowed to stand for an hour to permit the diffusion of the extracts and were subsequently incubated at 37°C for 24 hours. After the incubation period, the diameter of the inhibition zone was observed and measured in millimeters as an indicator of antibacterial activity [10].
Antifungal assay
To assess the antifungal properties of the leaf extracts from Parthenium hysterophorus, sterilized Sabouraud's dextrose agar (SDA) plates were utilized. Using a sterile cork-borer with a diameter of 6 mm, wells were created in the agar medium. In separate plates, 50 μL of ethanol, methanol, and ethyl acetate extracts were introduced into distinct peripheral wells. Additionally, a fungal disc was placed in the central well. To establish a baseline, a negative control (using DMSO) was included in one of the peripheral wells. The plates were then incubated at 28°C.
Following an incubation period of 3 to 5 days at 28°C, the inhibition zones around the wells were observed and measured in millimeters. These measurements were recorded to quantify the extent of antifungal activity exhibited by the extracts [10].
RESULTS AND DISCUSSION
Antimicrobial activity evaluation was conducted using a selection of five bacterial species, a yeast strain, and four fungal species. The method employed to gauge antimicrobial effectiveness was the agar well-diffusion technique, which targeted three Gram-positive bacterial strains and two Gram-negative bacterial strains. The outcomes revealed that the leaf extracts exhibited antimicrobial action against both Gram-positive and Gram-negative bacteria.
The extracts showcased their highest efficacy against Bacillus subtilis, followed by Pseudomonas aeruginosa, resulting in inhibition zone diameters (IZD) of 27.5 mm and 25 mm, respectively (as presented in Table 1).
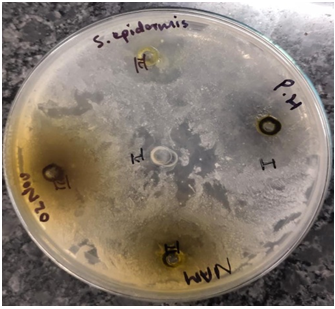
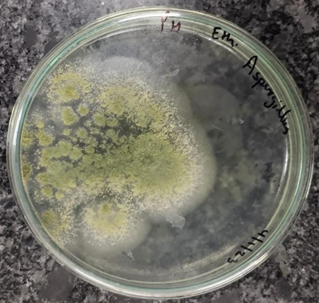
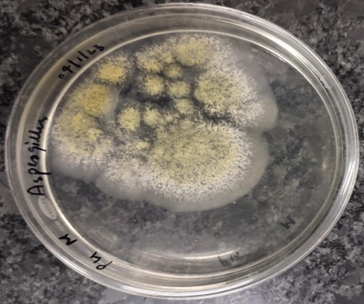
Figure 1-6 Zone of antibacterial inhibition of Parthenium hysterophorus leaf extracts against (A) Escherichia coli, (B) Bacillus subtilis, (C) Staphylocuccus aureus, (D) Staphylococcus epidermis,(E) Pseudomonas aeruginosa (F) Candida albicans.
A perusal of the study reveals that highest zone of inhibition against Escherichia coli was showed by methanol extract (22.5 mm) followed by ethyl acetate extract (17.5 mm) while ethanol extract exhibited no inhibition at all.
Figure 1. Escherichia coli
The methanolic leaf extract exhibited active inhibition with the highest zone against Bacillus subtilis of 27.5 mm followed by ethyl acetate (22.5 mm).
Figure 2. Bacillus subtilis
Among the other tested bacterial pathogens, highest zone of inhibition against Staphylocuccus aureus was exhibited by ethanol extract (20 mm) followed by methanol extract (15 mm) while against the Pseudomonas aeruginosa and Staphylococcus epidermis, maximum growth was inhibited by 50% ethanol extract (25 mm) and methanol extract (20 mm) followed by ethyl acetate extract (17.5 mm) and ethanol extract (18.5 mm) respectively.
Figure 3. Staphylococcus aureus
Figure 4. Staphylococcus epidermis
Figure 5. Pseudomonas aeruginosa
The only tested yeast, ethyl acetate extract exhibited maximum zone of inhibition against the Candida albicans (23 mm) followed by ethanol extract (17.5 mm).
Figure 6. Candida albicans
Table 1. The mean value diameter of inhibitory zones (mm) with Parthenium hysterophorus Leaf extracts and one negative control (DMSO)
|
Bacterial species |
Ethanol |
Methanol |
Ethyl acetate |
50% Ethanol |
DMSO |
|
Escherichia coli (NCIM 5346) |
_ |
22.5 mm |
17.5 mm |
5 mm |
_ |
|
Bacillus subtilis (NCIM 2920) |
12.5 mm |
27.5 mm |
22.5 mm |
10 mm |
_ |
|
Staphylocuccus aureus (NCIM 5345) |
20 mm |
15 mm |
12.5 mm |
10 mm |
_ |
|
Pseudomonas aeruginosa |
12.5 mm |
15 mm |
17.5 mm |
25 mm |
_ |
|
Staphylococcus epidermis |
18.5 mm |
20 mm |
12.5 mm |
17 mm |
_ |
|
Candida albicans |
17.5 mm |
11 mm |
23 mm |
15 mm |
- |
Percentage of Inhibition (PI) = C-T/C*100
Where C= Radial growth of the pathogen in control
T= radial growth of the pathogen in treatment
Table 2. The mean value of PI (in %) with the three leaf extracts and one negative control
|
Fungal species |
C = growth of pathogen in control (DMSO) in mm |
T = growth of pathogen in treatment in mm |
Percentage of inhibition (PI) |
||||
|
Ethanol extract |
Methanol extract |
Ethyl acetate extract |
Ethanol extract |
Methanol extract |
Ethyl acetate extract |
||
|
Fusarium oxysporum (NCIM 1008) |
90 |
30 |
30 |
35 |
66.67% |
66.67% |
61.11% |
|
Penicillium citrinum (NCIM 1435) |
25 |
20 |
20 |
NI |
20% |
20% |
- |
|
Rhizopus stolonifera (NCIM 1139) |
90 |
45 |
60 |
60 |
50% |
33.34% |
33.34% |
|
Aspergillus flavus (NCIM 1316) |
90 |
60 |
60 |
NI |
33.34% |
33.34% |
- |
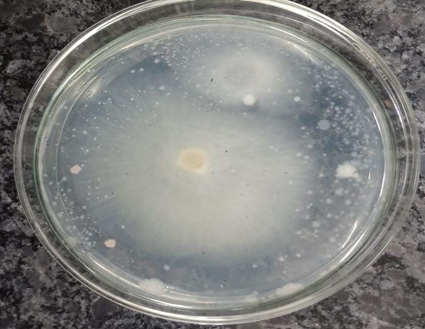
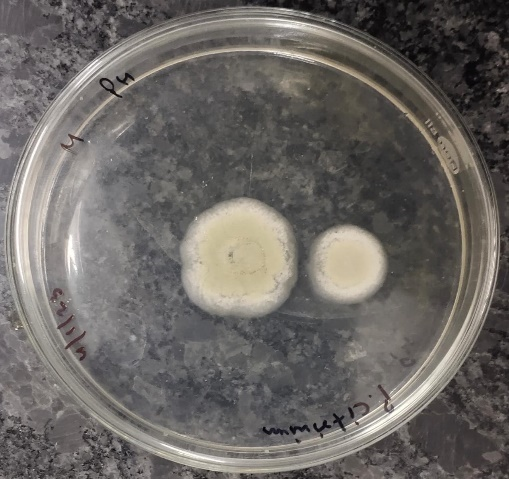
From the above observation we can say that ethanol extract exhibited active inhibition against the fungi Fusarium oxysporum, Penicillium citrinum, Rhizopus stolonifera and Aspergillus flavus with the percentage of inhibition (PI) of 66.67%, 20%, 50% and 33.34% respectively. Methanol extract also showed active inhibition against the fungi Fusarium oxysporum, Penicillium citrinum, Rhizopus stolonifera and Aspergillus flavus with the percentage of inhibition (PI) of 66.67%, 20%, 33.34% and 33.34% respectively. While ethyl acetate extract exhibited active inhibition only against the fungi Fusarium oxysporum and Rhizopus stolonifera with percentage of inhibition (PI) 61.11% and 33.34% respectively.
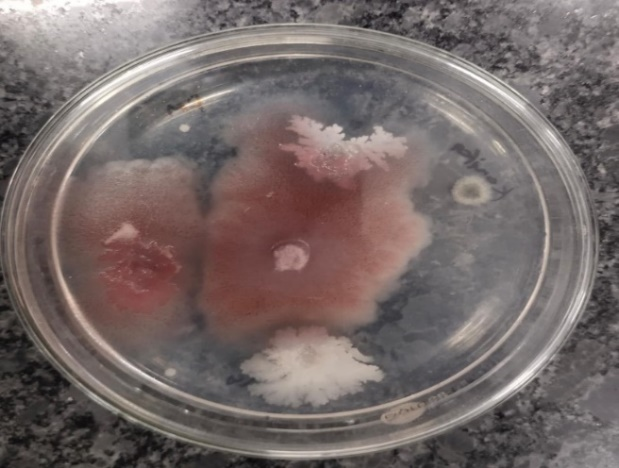
Figure 7A. Control- Fusarium oxysporum
Figure 7B. Fusarium oxysporum- Ethanol
Figure 7C.Fusarium oxysporum Methanol
Figure 7D. Fusarium oxysporum- Ethyl acetate
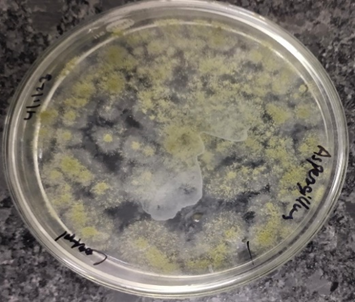
Figure 8A. Control–Aspergillus flavus
Figure 8B. Aspergillus flavus- Ethanol
Figure 8C. Aspergillus flavus Methanol
Figure 9A. Control-Rhizopus stolonifer
Figure 9B. Rhizopus stolonifera-Ethanol
Figure 9C. Rhizopus stolonifera–Ethyl acetate
Figure 9D. Rhizopus stolonifera – Methanol
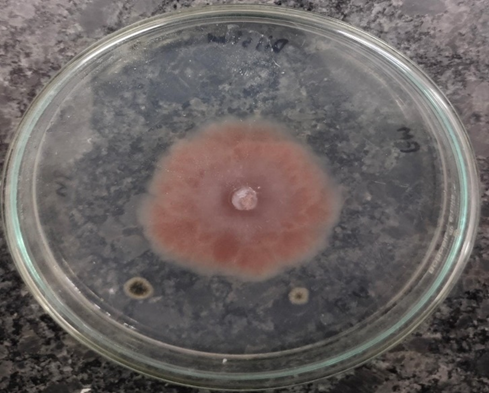
Figure 10A. Control- Penicillium citrinum
Figure 10B. Penicillium citrinum – Ethanol
Figure 10C. Penicillium citrinum- Methanol
Fusarium oxysporum was found to be the most sensitive among the all tested fungi and it was also found that ethanol and methanol are the most effective solvents for the extraction of bioactive compounds from the Parthenium hysterophorus.
There are very few investigations reporting the antibacterial and antifungal properties of parthenium. The antimicrobial efficacy of P. hysterophorus has been reported by various scientists as: Escherichia coli [9], Bacillus subtilis [11], Staphylococcus aureus [12], Pseudomonas aeruginosa, Candida albicans [13], Aspergillus flavus [14].
The effectiveness of plants in combating microbes is commonly attributed to constituents such as tannins, saponins, phenolic compounds, essential oils, and flavonoids [6]. In the case of Parthenium, its antimicrobial prowess is potentially owed to the presence of five terpenoids, volatile oils, flavonoids, as well as amino acids, sugars, and phenolic derivatives [15].
The assessment of Parthenium hysterophorus leaf extracts for their antimicrobial potential revealed varying levels of activity against the tested bacterial, yeast, and fungal species (as detailed in Tables 1 & 2).
Plant-derived antimicrobials hold significant therapeutic promise, being both cost-effective and environmentally friendly. Moreover, they tend to lack the side effects often associated with synthetic antimicrobials. Parthenium hysterophorus stands as a noteworthy addition to the medicinal plants exhibiting potent antimicrobial properties [16,17].
This current study differentiates itself from prior research in that it gauged antimicrobial activity across four distinct organic extract residues of Parthenium hysterophorus. Furthermore, this study serves as a stepping stone for future investigations aimed at identifying specific bioactive compounds that could potentially offer an alternative to chemical pesticides, thereby contributing to sustainable agricultural production.
CONCLUSION
The main objective of the aforementioned study was to evaluate the effectiveness of Parthenium hysterophorus leaf extracts in combating selected plant pathogens through antibacterial and antifungal actions. The utilization of plant-derived biocides in agriculture has garnered increased attention historically, primarily due to their minimal health risks and practicality.
The findings of this study substantiate that the leaf extracts from the Parthenium hysterophorus plant possess noteworthy antibacterial and antifungal attributes against the range of tested bacterial and fungal strains. Thus, based on the outcomes of this investigation, it can be reasonably deduced that Parthenium hysterophorus demonstrates substantial antibacterial and antifungal efficacy. These findings provide a foundation for further exploration, suggesting the potential for utilizing Parthenium hysterophorus as an alternative to chemical pesticides, thereby promoting enhanced and sustainable agricultural production practices.
ACKNOWLEDGEMENTS
The authors are grateful to Department of Science & Technology, DST-SERB under ASEAN-India S&T Development Fund (AISTDF) [Grant no. CRD/2020/000203] and are obliged to the funding agency for the financial support. The authors are also thankful to Dr. Ashok K. Chauhan, Founder President, Dr. Atul Chauhan, Chancellor and Prof. Dr. Balvinder Shukla, Vice Chancellor, Amity University- UP, Noida, for their motivation and research facilities.
CONFLICT OF INTERESTS
Authors declare no conflict of interests.
REFERENCES
- Luken JO, Thieret JW. (1997). Assessment and management of plant invasions. Germany: Springer Science & Business Media.
- Lalita KA. (2018). Review on a weed Parthenium hysterophorus (L.). Int J Curr Res Rev. 10(23).
- Adkins S, Shabbir A. (2014). Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag Sci. 70(7):1023-1029.
- Datta S, Saxena DB. (2001). Pesticidal properties of parthenin (from Parthenium hysterophorus) and related compounds. Pest Manag Sci. 57(1):95-101.
- Mtenga NC, Tarimo TM, Ndakidemi PM, Mbega ER. (2019). Carrot-weed: a noxious plant that threatens biodiversity in Africa. Amer J Plant Sc. 10(03):433.
- Bagchi AN, Raha AN, Mukherjee PR. (2016). A complete review on Parthenium hysterophorus Linn. Inter J Recent Advance Pharmaceutical Res. 6(1):42-49.
- Ramos A, Rivero R, Victoria MC, Visozo A, Piloto J, Garcıa A. (2001). Assessment of mutagenicity in Parthenium hysterophorus L. J Ethnopharmacol. 77(1):25-30.
- Kaur M, Aggarwal NK, Dhiman RJ. (2016). Antimicrobial activity of medicinal plant: Parthenium hysterophorus L. Res J Med Plant. 10(1):106-112.
- Madan H, Gogia S, Sharma S. (2011). Antimicrobial and spermicidal activities of Parthenium hysterophorus Linn. and Alstonia scholaris Linn. Indian J Nat Products Resources. 2(4):458-463.
- Bezuneh TT. (2015). Phytochemistry and antimicrobial activity of Parthenium hysterophorus L.: A review. Sci J Anal Chem. 3(3):30.
- Fazal HI, Ahmad N, Ullah I, Inayat H, Khan L, Abbasi BH. (2011). Antibacterial potential in Parthenium hysterophorus, Stevia rebaudiana and Ginkgo biloba. Pak J Bot. 43(2):1307-1313.
- Barsagade NB, Wagh GN. (2010). Comparative screening of leaf extracts of common plants and weeds for their antibacterial and antifungal activities. Asiatic J Biotechnol Resources. 3:227-232.
- Malarkodi E, Manoharan A. (2013). Antifungal activity of Parthenium hysterophorus L. J. Chem. Pharmaceutical Res. 5(1):137-139.
- Kumar S, Khandpu S, Rao DN, Wahaab S, Khanna N. (2012). Immunological response to Parthenium hysterophorus in Indian patients with Parthenium sensitive atopic dermatitis. Immunol Invest. 41(1):75-86.
- Kumar A, Joshi S, Malik T. (2013). Antimicrobial potential of Parthenium hysterophorus Linn plant extracts. Inter J Life Sc. Biotechnol. Pharma Res. 2(3):232-236.
- Bagchi AN, Raha AN, Mukherjee PR. (2016). A complete review on Parthenium hysterophorus Linn. Intern J Recent Advance Pharmaceutical Res. 6(1):42-49.
- Cowan MM. (1999). Plant products as antimicrobial agents. Clin Microbiol Rev. 12(4):564-582.
.png)
.png)
.png)