Previous Issues Volume 9, Issue 1 - 2025
Individual Stringent Mini Isolators for Autologous Cell Therapy
Didier Meyer*
52 Ter Avenue de Saint Cloud 78000 Versailles (F), France
*Corresponding author: Didier Meyer, 52 Ter Avenue de Saint Cloud 78000 Versailles (F), France, Cell Phone: 33(0)607504088, Email: [email protected]
Received Date: September 25, 2023
Published Date: February 28, 2025
Citation: Meyer D. (2025). Individual Stringent Mini Isolators for Autologous Cell Therapy. Mathews J Pharma Sci. 9(1):45.
Copyrights: Meyer D. © (2025).
ABSTRACT
The Chimeric Antigen Receptor of T cells (CAR-T) allows through a leukapheresis, a transduction and a growth of cells to create “killer cells" against a dedicated tumour of a patient which is the donor in case of autologous CAR-T. This article presents one individual decentralised solution for solid tumours with a mix of technologies (RTP isolator, Single Use Systems, CPC connectors) at an affordable price to prevent from any contamination during the whole process before the injection of the dedicated “killer cells” to the patient.
Keywords: Autologous Cell Therapy, Blood, Environment, Patients
THE PRINCIPLE
Autologous Cell Therapy (CT) is the paragon of what anyone could expect in case of cell disorder, individual blood collection (3 to 5 hours of apheresis), cells separation, cell identification and selection, cells modification before expansion and injection to the patient who is the donor of the reengineered cells without any problem of blood compatibility. The details of the working process to succeed are described in a schematic provided by Thermo Fisher:
Recent advances with CAR-T allow to produce and expand “killer cells” dedicated to one specific tumor in one to three weeks.
The prices of these protocols are between 300 to 400 K$ which make them difficult to be reimbursed and to be considered as a treatment of first intention That limits their prescription and consequently the progress and the implementation of this new technology.
The pricing goal to be compatible with a reimbursement around 50 K $ requests a rationalization of the process, its environment and the locations of the decentralized platforms in the vicinity of the patients to avoid storage, freezing and thawing which affect the quality of the cells at the final infusion to the patient
This very level of price would allow to propose this technology as a first intention treatment and so to multiply its uses.
THE COMPLIED DECENTRALIZED PLATFORMS
The environment in which these handlings must allow to inject to the patient a sterile and particle free infusion according to the current Good Manufacturing Practices (cGMP) means that beyond the research and development there must be a logic of producer to implement an installation fitting with specific worldwide regulations.
In the recent EMA revised annex 1 (The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use of August 22, 2022)
which has been approved by PIC/S, ICH and WHO there are 2 main choices of environment to isolate an aseptic bio-pharmaceutical process from its immediate surroundings either a class A working station like a Bio-Safety Cabinet in a class B surrounding or n dedicated isolator to this application within a class C or D surrounding. These A, B, C and D classes mean not only a defined ventilation/filtration, a speed of air and a filtered air renewal but a specific gowning for the personnel with a time for a mandatory gowning with an assessed training for its 4 times a day practice.
The average times given are 5 minutes for the access in case of class C or D and 20 minutes for the access in class B which can be considered as a non-productive but mandatory “waste” of time.
For the comfort of the operator and the efficacy of the segregation between personnel and process the choice of isolator with a class C or D surroundings according to local regulation eases the daily works.
THE ISOLATOR
Closed isolators with flexible or rigid walls have been described and used from the late 40s in sterile surgery (1949-US PATENT OFFICE N° 2473033 STERIE SURGERY) and in breeding of lab germ-free rodents. Since the last 30 years they are routinely used in the bio-pharmaceutical industry.
The Revised Annex 1 which classifies 3 segregation technologies (A in B, RABS and isolators) published August the 22nd 2022 to be applied one year later and 2 years later for the lyophilized products) gives the technological and the gowning details.
AN EXAMPLE OF ISOLATOR FOR MULTIPLE CELL THERAPIES
A specific isolator for Cell Therapy with integrated lock chamber for transfer of components with incubator and centrifuge has been presented to the market by Sanyo in 2016. This type of equipment fits perfectly for the research and development of sequences to be then applied in daily routine.
INDIVIDUAL MINI ISOLATOR FOR EACH PATIENT
In case of multiple Cell Therapy (CT) process each individual process must be separated and autonomous.
Conversely from the above CT Sanyo isolator which can be used for several patients at the same time the individual mini isolator is dedicated to one patient and so avoids any risk of cross contamination and confusion in protocols.
One possible solution to keep the segregation during all the whole process is the use of individual flexible mini-isolator, which is a variation of what has been published by INRA (French National Institute for Agronomical Research) for studies with mono contaminated mice allowing in the same room several individual isolators with different mono contaminations without any risk of cross contamination.
To keep individual autologous CT separated process a similar solution is a flexible sterile closed isolator, single and ready to use, which is Gamma sterilized as a Single Use System (SUS). The advantage of a flexible enclosure is the easiness of modifying its design in case of adding new items or gimmicks to facilitate the process.
THE FOLLOWED MODEL OF CLOSED ISOLATOR from a publication of 1979 (in French)
With as a key element: a Beta RTP transfer system
The Beta part of the RTP system of the mini-isolator is molded by injection. Its price is compatible with a Single Use System (SUS).
A similar system is used for cartilage reconstitution by Codon in Leipzig as a door for incubator.
At least 6 companies (2 German, 2 French, 1 Swedish and 1 UK) are manufacturing and selling this type of system.
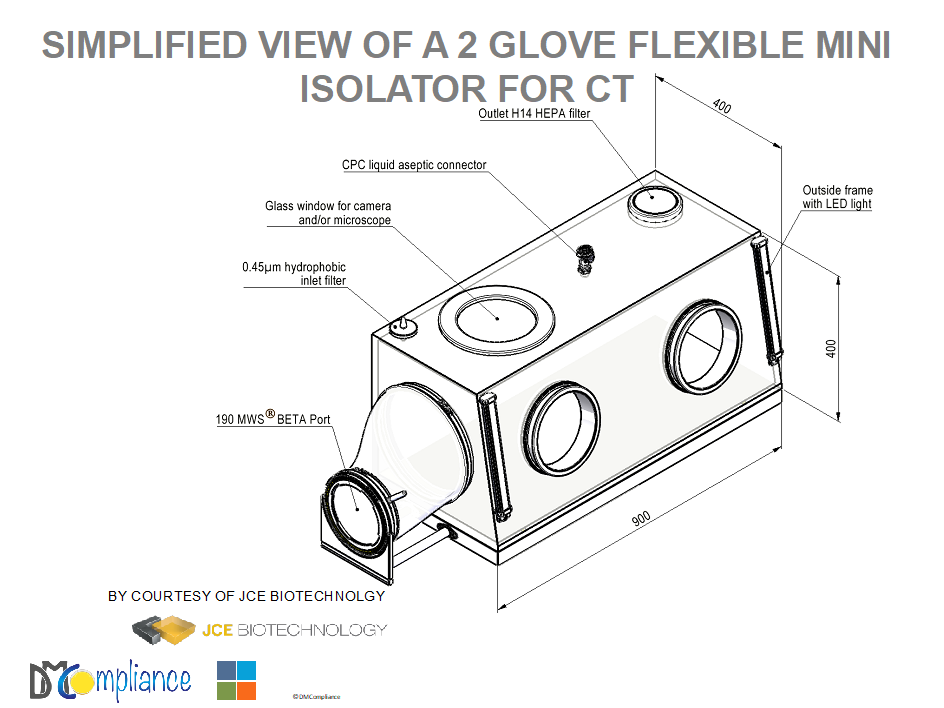
A PROPOSED DESIGN FOR AUTOLOGOUS CELL THERAPY:
ERGONOMICS
- 250 l Gamma sterilized isolator made of a flexible transparent film
- Glass or equivalent view from the top as a surgeon on the top of the wound
- Transparent glass zone on the top for microscope or camera for slides
- RTP Beta door
- Inlet 0,45-micron hydrophobic micro-filter
- Inflated with prefiltered compressed gas (air or a specific gas to help the growth of cells)
- Outlet HEPA filtration to avoid any return of airborne contamination
- Genderless sterile connector(s) for sterile transfer of liquid(s) (CPC)
- Two adjacent forearm glove/sleeve systems
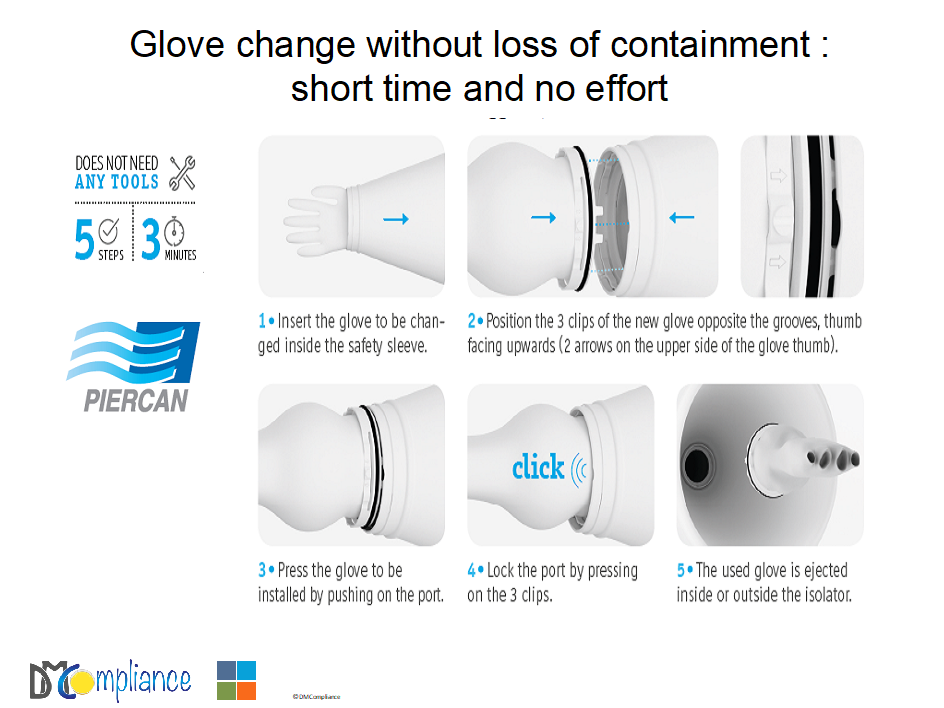
- Gloves with a safe glove change for proper size and accident (BCS by PIERCAN)
- Gamma sterilization of the whole system
- Possibility of foot pedal action
WITH THESE FEATURES
- Sterile & Ready to use as Gamma Sterilized
- A thin canopy 0,5 mm allowing WIFI, magnet effect and room temperature to go through
- Manufactured in ISO 8 conditions to avoid spreading of particles
- Easy identification from the outside with bar coded or equivalent components
- Transparency with a perfect view from the outside
- Flexibility of the base to create motions during the expansion phase
- Immediate access and height adjusted support to fit with each operator
- Glove safe change to choose the proper size and thickness
- Trolley to move from 20° to 35° C closets/rooms
- No blower, no noise, minimum consumption of energy
- Adjusted cold lighting from LED in outside frame
- Canopy with extra volume for sealed safe collection before infusion to patient
GLOVE SAFE CHANGE TO FIT WITH USER’S HANDS
In isolators, thanks to attached gloves, operators are physically inside but biologically outside. The size of the gloves usually between 6 to 8 must fit with operator’s hand.
PROTOCOLS
All items and materials to be introduced in the proposed Class C or D cleanroom are bar coded or equivalent, sterile and double wrapped before to go through a H2O2 lock chamber after removing the first wrapping.
To avoid a freezing/thawing process of the cells a decentralized implementation of identical platforms for 5 mini isolators allow to take care of 80 to 240 patients per year. The cycle between the blood collect to the infusion of modified cells for each patient takes between one to three weeks, which is always too long for the patient. So, all the techniques to speed up the process are welcome. Any modification of the design only needs an easy change of the design of the flexible canopy.
CAMERA AND/OR MICROSCOPE
Outside of the isolator thanks to a glass panel from its top for a clear view, a camera and/or a microscope can be installed to check periodically the evolution of the cells.
CPC ASEPTIC CONNECTORS FOR LIQUIDS AND RTP TRANSFER FOR ITEM
The genderless aseptic connectors by CPC are used to transfer blood and cells without any risk of contamination. They cannot be disconnected, and clamps are required to close the liquid pathways.
In the case of RTP connection for solid items multiple connections and disconnections are possible and easy to perform. This system has been patented in 1962 under its original acronym of DPTE by the CEA (French Atomic Energy Board).
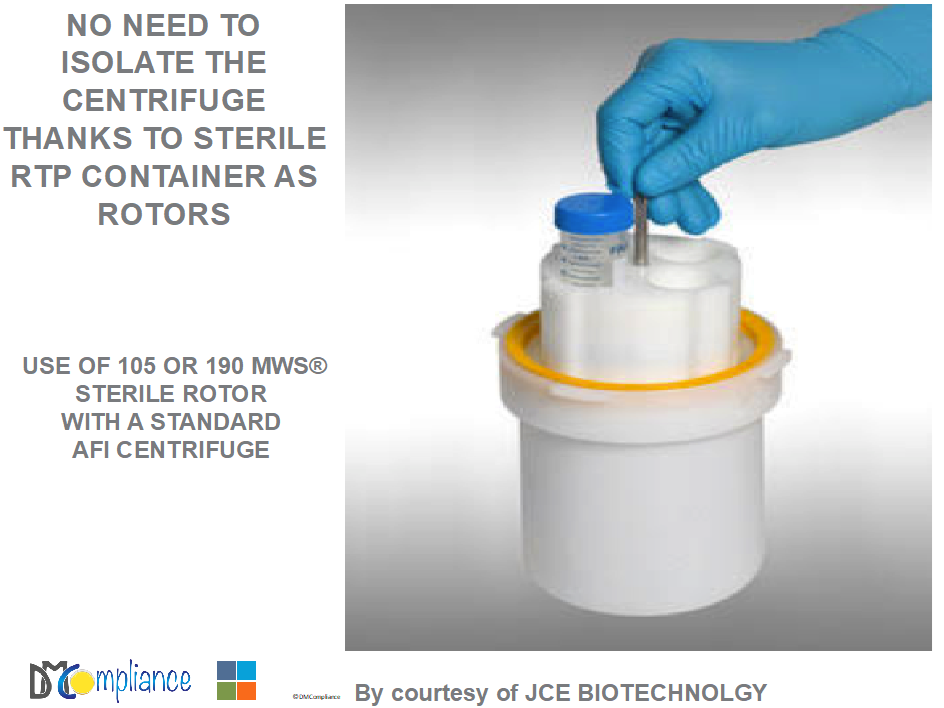
THE USE OF RTP CONNECTION FOR A CENTRIFUGE
Conversely to the Sanyo example, with the use of RTP container the centrifuge doesn’t need to be isolated and bio-decontaminated, only the Beta RTP container is Gamma sterilized and used through a service isolator.
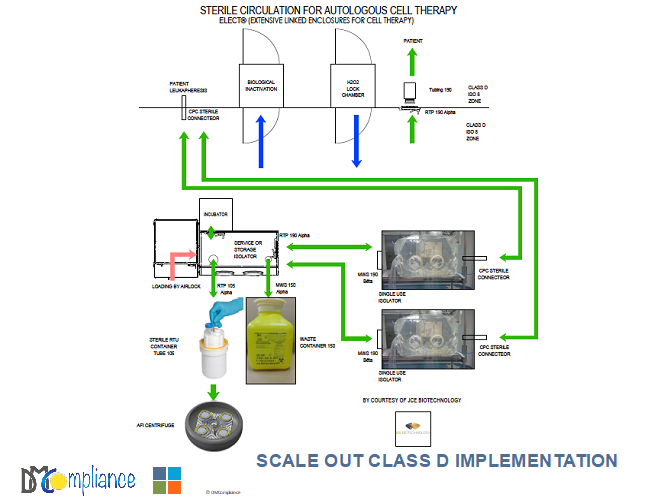
THE DECENTRALIZED PLATFORM
The platform (s) can be in hospital or blood bank near the patients. Their environment is a class D or C. Their footprint fits for 5 mini-isolators plus 2 in preparation. Each platform is headed by a scientist with 2 technicians for logistics and computerization.
COMPUTERIZATION
Each of the identical platform is autonomous for cyber security and must comply with the GAMP 5 CFR 21 11 for its e-organization:
- Computerization of all process
- E-Identification of all the protocols, equipment and items
- The prepared infusion to the patient must be sterile and cannot be submitted to a sterility testing so must fit with a RT/RT
E-Protocols with Real Time Release validations (EU 2018 ANNEX 17: Real Time Release Testing and Parametric Release):
3.1 Under RTRT, a combination of in-process monitoring and controls may provide, when authorized, substitute for end-product testing as part of the batch release decision. Interaction with all relevant regulatory authorities prior and during the assessment process preceding regulatory approval is required. The level of interaction will depend on the level of complexity of the RTRT control procedure applied on site.
WASTE
The waste consists in all the parts and products which are not the patient’s infusion.
This biological waste must be inactivated before to be incinerated outside.
For instance, a two-door autoclave at 90°C can be used.
ECONOMICS
Each platform is equipped with:
- A Centrifuge using RTP containers (105 or 190) as rotors (AFI/ JCE Biotechnology)
- RTP Centrifuge service isolator to go from a vertical to an horizontal transfer for the collection before and after centrifugation
- H2O2 bio-decontamination lock chamber to enter double wrapped sterile items in class D
- RTP Storage and bio-decontamination isolator for the identification/protocols, the sterile transfer and the handling of all the necessary single wrapped items
- RTP Service transfer isolator when needed
- Intermediate control of the cells with a microscope and/or a camera
- Transfer to the patient room of the final infusion through a Beta RTP sleeve
- Biological inactivation of all the waste before incineration outside
ENVIRONMENT CAPEX FOR EACH PLATFORM
- 70 m2 of Class D room: 180 K€
- Storage isolator: 250 K€
- Service isolator: 150 K€
- Centrifuge service isolator: 150 K€
- Note: Each isolator includes its own H2O2 biodecontamination and ventilation/filtration systems
ENVIRONMENT OPEX FOR EACH PATIENT
Individual mini-isolator: 8 K€
Sterile centrifuge RTP container: 600 €
Sterile waste RTP container: 300 €
1/30 sterile RTP tubing: 50 €
TO CONCLUDE
The use of isolators as an environment option saves time & money and its management is easier & safer than a conventional A in B design.
This avoids any cross contamination and mistakes.
E-identification of all the steps is an essential part of the protocols.
The decentralized identical platforms can be implemented in the vicinity of the patient’s hospitals or blood banks to perform the autologous CT more easily.
The development of the technology of CAR-T for autologous CT will allow to constantly improve the future cures of patients.
.png)
.png)
.png)
.png)
.png)