Previous Issues Volume 6, Issue 1 - 2021
Genetic Variants associated with Persistent Depressive Disorder
Ramakrishnan V*, Lenika A
Human Cytogenetics and Genomics Laboratory, Faculty of Allied Health Sciences, Chettinad Hospital and Research Institute (CHRI) Chettinad Academy of Research and Education (CARE), Kelambakkam-603103, Tamilnadu, India
*Corresponding Author: Dr. Ramakrishnan V ,Faculty of Allied Health Sciences, Chettinad Academy of Research and Education, Chettinad Health City, Kelambakkam - 603 103, Tamil Nadu, India, E-mail: [email protected]
Received Date: November 09, 2020
Published Date: February 05, 2021
Copyright: Ramakrishnan V, et al. © (2021).
Citation: Ramakrishnan V, et al. (2021). Genetic variants associated with Persistent Depressive Disorder. Mathews J Psychiatry Ment Health. (6)1:31.
ABSTRACT
Background and objectives: As with depression, persistent depressive disorder, recognized as dysthymia is one of the common psychiatric illnesses. According to the World Health Organization, over 350 million among the entire population, are affected by this condition. This study intends to cover and narrate the association of candidate genes with persistent depressive disorder.
Methods: A well-focused subject literature search should be chosen for this study from PubMed and PubMed Central literature review or narrative review is a type of review articles are used to focus on genetic variants are expected to have only minute effects on the whole disease risk, and numerous genetic factors amongst with environmental factors are possibly required for the progress and developing of major depressive disorder.
Results: Genetic association of a depressive disorder is vital for clinical-based studies, and also many researchers have suggested that common genetic variants, gene expression and pathways are related to depression. Thus, it is established those candidate genes are involved in the pathological process of depressive disorder such as CRY1, CRY2, MAOA, 5-HTT; BDNF, IL, and FKBP5 are mainly concerned with anxiety and depressive disorders.
Conclusion: To conclude, our findings in genetic literature confirms that association studies of candidate genes point to potential risk loci of persistent depressive disorder may play an important role in early stage identification and pathogenic risk assessment, thereby reducing the incidence among the PDD human community. Depression can be treated efficiently and easily but the main issue is to find out the associated risk factors and severity of the depression.
KEYWORDS: Persistent depressive disorder; Candidate genes; SNPs; Genetics, Environment
INTRODUCTION
Psychiatric disorders are described as a severe form of depression such as clinical depression, bipolar disorder (BD), and schizophrenia (SCZ) that are linked with higher rates of health problems throughout a person's life. The incidence of Major Depressive Disorder (MDD) among sufferers with a physical sickness is reported to be approximately twofold to threefold greater in the worldwide population and comorbidity with physical ailments such as stroke; diabetes, cardiovascular disease, pain, and malignancy have become a global health concern [1-3]. It is expected that one of three depressed patients might develop unfavorable persistent depression [4].
Persistent depressive disorder (PDD), recognized as dysthymia is described as a depressive mood and is not severe as the leading depression, however, it is chronic. It happens particularly as women often are assigned lower levels of ability than men [5]. DD (Dysthymic disorder) is categorized into mood disorder characterized by persistent depression along with any of the two symptoms: eating disorders (overeating or less appetite), sleep difficulties (insomnia or hypersomnia), attention deficiencies (poor decision-making abilities and concentration difficulties), and hopelessness [6]. The widespread comorbid condition with dysthymia in kids and teenagers are anxiety disorders, conduct disorder, learning disorders, attention deficit hyperkinetic disorder, and mental deficiency. DD has higher comorbidity with other psychological disorders, this serves as the reason for the misinterpretations during the screening of dysthymia or PDD [7,8]. In the 1980s, the prevalence of dysthymia in the U.S. as presented by a survey was 3% (in the adult population) and its comorbidity with other mental illnesses as estimated was 25%-30% [9]. When the GBD analysis has been carried out to determine the burden of different depressive disorders in the year 2010, depressive disorders were reported to be the second main attributor to YLDs (years lived with disability) among which dysthymia accounted for an average of 1.4% of YLDs and 0.5% of DALYs (disability adjusted life years) [10].
According to Vandeleur et al, the estimated prevalence of DSM-5 PDD with 1) persistent major depressive episodes is 15.2%, and 2) pure dysthymia is 3.3%. Most times, dysthymia moderately responds according to treatment, although some residual symptoms remain, which the potential risk factors are for relapse [11,12]. Recent research on treatment outcomes of dysthymic patients suggests that the combination of psychotherapy and pharmacotherapy has the best outcome [13].
RISK FACTORS OF DYSTHYMIC DISORDER
Familial inheritance of dysthymia is one important factor considered in studies of dysthymia. Generally, the family history of mood or psychological disorders was recognized as an important contributor to dysthymia. The occurrence of dysthymia (DSM-III) in children whose parents were diagnosed with primary unipolar major depression was much higher compared to children of parents with no history of psychological disorders. Higher rates of major persistent depressive disorder in relatives of both dysthymia and MDD patients were observed [14]. A higher risk of familial personality disorders was also related to dysthymia, where the more the relatedness to the affected individual, the higher is the risk of dysthymia [15]. Various degrees of childhood adversity were also reported to be predictors of dysthymia and co-morbid dysthymia [16 and 17]. Experiencing stressors in early life affects the levels of neurochemicals which might favor the development of dysthymia [18]. Figure 1 explains the major risk factors of depressive disorder.
Figure 1: Major risk factors of depressive disorder.
FUNDAMENTAL GENETIC CONCEPTS
Based on the results of the Human genome project, nearly 20,000 nuclear genes were identified and most of them function in the neuronal region (brain). Several traits are the reflection of many genes and environmental factors. The accountability of gene expression still while genes are present, they do not always express themselves in the same way as the complexities of this construct have important implications of studying mental disorders. Traditional genetic studies are fairly useful, where the evidence from these studies is the existing support for the genetic foundation of psychological disorders. This investigation produces the baseline of genetic influence, distinguishing the comparative influence of heredity from that of the environment [19]. But there is a limit to what common genetic studies can reveal. They cannot recognize a specific gene defect. Because of this limitation, researchers have been eager to apply the innovative, powerful tools of modern genetics to the study of psychological disorders.
GENETICS OF BEHAVIOR AND PSYCHOLOGICAL SICKNESS
The purpose of molecular genetics research of psychological disorders is to classify and express cloned genes that contribute to the risk of particular disorders and influence their way. However, the connection between genes and mental disorders is complex and intricate to assess [20,21]. Analysis about genetic associations is a choice in conformity with linkage studies and one of the greatest strategies for identifying the genes accountable for genetically multifaceted diseases in which there is no recognized inheritance model, in which several genes with small effects are most likely involved. The discoveries at the genetic level should prove to transform influence on clinical practices. However, behavioral and mental illness concerning genetics is complex. This is consistent with the intricate wiring of the brain and the variations in human behavior, however the capability to that amount the advantages of genetics because of action continues to be some years.
SNP detection and associating it with psychological disorders
The implementation of vital technological platforms for facilitating gene invention is the success of the global Human Genome Project and similar projects within the industry. They aim to inform the genome sequence conditions and several international projects aimed at identifying deviation in all human genes among individuals. The major focus of variations in recent studies is a gene sequence difference where a single base is altered [22]. The SNP analysis is fairly easier to recognize the locus of a human gene that contributes very larger effects on phenotype than to recognize several loci where each contributes a minute effect [23]. Over the past years, genome wide association studies, commonly known as GWAS analyzes the common SNPs and copy number variations (CNVs) and have quickly substituted the candidate genes in association studies (Figure 2). However, the identification of genetic variants in MDD vulnerability and other depressive disorders is quite challenging [24]. Several Candidate gene approaches versus GWAS studies observed depression and gene association, yet DD or PDD is not widely studied. Several reasons behind the lesser number of studies include the diagnostic difficulty, the comorbidity of the disorder (double depression cases), and the similarity with MDD (Major Depressive Disorder). The technological advances including molecular methods and high-throughput analytical techniques are potential for assessing every individual gene as a viable candidate gene for the risk of psychological disorders, or definitely, other phenotypes of interest.
Figure 2: The general depiction of GWAS (case-control method).
Cryptochrome Circadian Regulator 1 and 2 (CRY1 and CRY2)
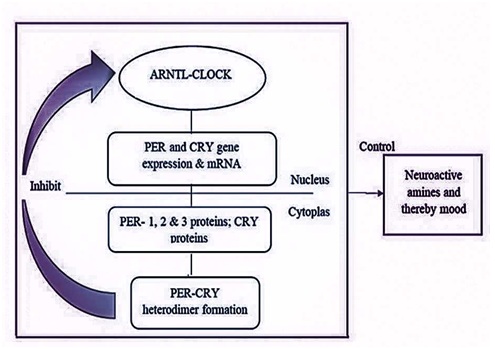
The molecular mechanism of circadian clocks in mammals involves the central SCN and peripheral clocks throughout the body. The duration of the circadian cycle depends on certain genes called clock genes such as CLOCK, ARNTL, PER, and CRY. The transcriptional efficiency of the clock genes is maintained by a natural light-dark based 24-hour rhythm mechanism [25]. The core genes such as ARNTL and CLOCK are mainly involved, and the binding initiates a feedback loop in which CRY and PER genes, and proteins play an imperative role. The transcriptional and translational events of the clock components are important for rhythm generation. Figure 3 explains the general feedback loop of the clock signals in the SCN (suprachiasmatic nucleus) that involves CLOCK, ARNTL, PER, and CRY2 genes and protein. The down-regulation of the mentioned cycle will affect the output pathways involving hormones, neuropeptides, mood, and behavior. As several studies report, the circadian clock period alterations are due to post-translational modifications in the clock genes. The disturbances in the circadian clocks and the expressional changes of the CRY1 and CRY2 genes were observed in psychological and mood disorders such as Bipolar disorder, winter depression, Major Depressive Disorder, sleep disturbances, and metabolic disorders. These former associations paved the way for researchers to find a possible association of these circadian cycle regulatory genes with PDD. CRY1 and CRY2 play a vital role in the circadian clock, and the ratio of CRY1 and CRY2 determines the life span of the circadian clock.
Figure 3: The feedback loop in SCN involving PER and CRY gene.
Researchers had found that 73% amino acid of the CRY1 human (6-4 photoproduct) photolyase is the same as that of CRY2. The purified human proteins, CRY1 and CRY2 are viewed as maltose-binding fusion proteins up to the expectation that encloses FAD and a pterin cofactor permanency [26]. Dysfunction of cell signaling pathways linking CRY1 and CRY2 may lead to depressive behaviors. Communication of these two proteins directly with the Gsα, a subunit of the heterotrimeric G protein [27], and the adenylyl cyclase pathway [28] decrease the aggregation of these sites. This communication of the CRY proteins is associated with downstream protein expression and function [29]. Based on the inhibition, the cyclic adenosine monophosphate signaling proceeds, leading to several downstream cascades including the cyclic AMP reaction element-binding protein action, and thereby contributing to depressive-like behaviors [30]. Increased CRY1-CRY2 ratio decreases the PER2-CRY2 import to the nucleus with its related proteins and this, in turn, lengthens the circadian period. The genetic association studies of dysthymia state that CRY2 and CRY1 are not associated with DD, but haplotype analysis reported rs10838524, rs7121611, rs7945565, and rs1401419 to be associated with dysthymia [31]. A follow-up study proved the association of all the former SNPs and rs3824872 [32].
Monoamine Oxidase A (MAOA)
The MAOA gene mapped in the X chromosome at p11.3 codes for monoamine oxidase A that functions in the metabolism of neuroactive or vasoactive amines such as dopamine, serotonin, and norepinephrine which were proven to be involved in several psychiatric illnesses [33]. The brain monoamine level is important for the brain’s motor cognition, mood, and emotion [34]. The MAOA gene polymorphisms such as rs1137070, 941T-G, and rs1799835, an intron-2 dinucleotide repeat, and functional length polymorphism in the upstream region of VNTR (u-VNTR) were observed to affect the in-vitro transcriptional efficacy of neuroactive amines and also in several behavioral and neuropsychiatric illnesses such as the PD, ADHD, MDD, BD, and AD. Studies report the significant relation of the MAOA genotypes with childhood maltreatment, abuse in depression. A study conducted by Cichetti et al., (2007) predicted that adolescents with low MAOA levels along with a history of maltreatments produce more depressive symptoms [35], while another MDD symptomatology study concludes the association of higher MAOA levels and abuse with MDD symptoms [36].
A study by Nikulina et al., in 2012 reported that the higher MAOA levels (MAOA - H) with childhood maltreatment and abuse as a vulnerable factor in females and the reasons behind the sex specificity have also been explained [37]. A study conducted in 2013 by Melas et al., reported the existence of depressive symptoms in MAOA – L (low MAOA levels) females with childhood maltreatments than those with MAOA-H thus is contentious to the previous study [38]. There are several studies relating MAOA genotypes to depressive symptoms and treatment, yet, other studies provide evidence for their nil association with depression and depressive symptoms. However, in the case of PDD or dysthymia there is a very less number of studies focusing on MAOA genotypes, yet, the result of Nikulina et al. has given hope for the possible association of this gene and its transcription levels in dysthymia. The conclusion of the exact role of this polymorphism in PDD or DD is in the hands of future studies.
SEROTONIN TRANSPORTER GENE (5-HTT)
The 5-HTTLPR (5-Hydroxytryptamine transporter-linked polymorphic region) is a 5’ proximal del-ins polymorphism which is linked with the transcriptional efficiency and decreased functioning of 5-HTT, which in turn, was related to altered serotonin uptake from the synaptic clefts. The s-allele carriers of 5-HTTLPR are observed with significantly lower transcription levels of the promoter than those with the L-allele and causing altered uptake of serotonin. As stated by several studies, the transcriptional efficacy of 5-HTT interferes with the mood tone, circadian cycle, sleep, motor function, sexual behavior, and appetite. The involvement of this gene polymorphism in some psychiatric disorders was not reported [39].
The 5-HTTLPR s-allele is accounted for higher risk of childhood onset depressive disorder [40], whereas a study in 2000 stated no nominal relationship between this polymorphism and dysthymia in Brazilians [41]. However, the study by Verhagen et al. investigated the polymorphism of 5-HTTLPR in MDD and its comorbid disorders and observed the comorbidity of dysthymia in 36.9% of MDD patients. The GEE analysis states that the comorbidity of dysthymia in s-allele carriers was significantly lesser compared with the l/l genotyped individuals [39]. Studies also reported the significant interplay between the polymorphisms in this gene and specific environmental factors such as childhood maltreatment and sexual abuse in depressive episodes [42]. Although a lesser number of studies investigated the direct effects of the serotonin receptor polymorphism in persistent depressive disorder there are MDD and recurrent depression studies that prove the possibilities of the involvement of this gene in PDD.
BRAIN-DERIVED NEUROGENIC FACTOR (BDNF)
Brain derived neurogenic factors are significant in neurogenesis, neuronal cell properties such as plasticity and extensibility. Depressive disorders and other neuropsychological disorders like Schizophrenia were related to decreased serum BDNF levels and Val66Met polymorphism. A study determined that the BDNF serum concentrations are higher in dysthymias than those of the MDD patients, and was also observed with a nominal difference when compared with controls [43]. In contrast, a study determined that the serum BDNF levels of dysthymic and patients with MDD are significantly lower than controls, and did not note any difference in the concentrations between the two depressed groups [44]. The existence of a correlation between Val66Met and mood disorders is studied in different populations, and also the methylation of BDNF is observed to affect the susceptibility of depressive disorders and its persistence in patients with the possible acute coronary syndrome [45]. The over presentation of Met allele (Val66Met) in BDNF was suggested to be linked with dysthymia in women who experienced childhood problems [46].
BDNF gene polymorphisms are documented in several studies including MDD, schizophrenia, childhood onset disorders, bipolar disorders and other mood disorders. However, we can observe contradictory outcomes in case of the risk of the mentioned conditions, whereas, some studies report these polymorphisms to have a potent role in the treatment and diagnosis of several mood disorders. In the case of dysthymia, although results were favorable to the relationship between BDNF levels and dysthymia there is a need for a much larger GWAS to elucidate the exact relationship of this gene with the risk of PDD.
INTERLEUKIN (IL)
The cytokines and their effect on mood are widely studied and data clarify a bi-directional relationship concerning the cytokines and mood disorders [47]. The cytokines are observed to influence neuroendocrine functioning and therefore will further affect the neurovegetative functions [48]. The increased production of IL-1β and IL-6 levels was observed in MDD and Dysthymia (cells induced by mitogen), the IL-1β levels are highly correlated with onset age and duration of the illness [49 and 50]. Yet, another study proved no correlating levels of IL-1β and the psychological parameter [51]. The haplotypes of IL-1 gene were studied in Brazilian cohorts and the result suggested several IL-1 haplotypes that are exclusive to dysthymic patients, these haplotypes include IL-1RA, IL1-β, and IL1-α which in several combinations might increase an individual’s risk of PDD [52]. Even though studies report altered levels of interleukins IL-1β, IL-6, IL-2 in PDD, until now, only one study investigated the interleukin haplotypes in the progression of PDD. However, the positive association of IL haplotypes with PDD was reported by Fertuzinhos et al., and therefore the replication of this in various populations can determine the efficacy and reliability of the result.
PROTEIN KINASE C DELTA BINDING PROTEIN (PRKCDBP)
The protein kinase C delta binding protein plays a role in synchronizing the circadian rhythm, where the synchronizing process involves the maintenance of a stable PER2-CRY2 complex. The abnormal expression of CRY2 results in increased expression of PER2 (period 2) and MAOA; the Nuclear receptor subfamily 1 group D member 1 (NR1D1) links the circadian clock and the mood events. The PER2 interacts with NR1D1 thereby resulting in a sequence of events by adipose tissue leading to lowered mood or depression. A gain of function mutation in the PRKCDBP thus seems to increase the circadian period, whereas, a loss of function mutation shortens the same. The association of the “A” allele of PRKCDBP rs1488864 was reported with MDD, depressive disorders in general or (and) dysthymia by Kovanen and colleagues. Although this study did not concern dysthymia in specific for the PRKCDBP rs1488864 polymorphism, its association with depression disorders (either depressive disorders (or) and dysthymia) was reported [OR=1.5 (1.2–1.8), p=0.0009]. The haplotypes of the PRKCDBP gene ATTA (rs1488864, rs2947030, rs16911940, rs10839553) was also observed to be considerably related to depressive disorders (OR=1.5 and p=0.001) and MDD [32]. Although this study by Kovanen et al. observed the association of this gene with depressive disorders (and dysthymia), there are several limitations concerned with this study among which the limited coverage of this gene is the main.
FK506 BINDING PROTEIN 5(FKBP5)
FK506 binding protein 5 is found to be connected with regulating the GR (glucocorticoid receptor) sensitivity of cortisol, and consequently, the FKBP5 gene expression affects the cortisol secretion. Increased FKBP5 expression will lead to decreased GR activity and thereby less efficient stress lowering mechanisms. This GR-cortisol signaling impairment seems to be an important biological attribute of mood disorders. The FKBP5 secretion alters GR activity, whereas, the FKBP5 expression is regulated by the binding of the Androgen Receptor. The study conducted by Velders et al., used candidate genes and GWAS for the detection of genes linked with diurnal cortisol secretion and their association with depression, they reported the association of rs9470080 with the saliva cortisol levels and clinical symptoms of depression. A Swedish population study on rs1360780 reported that the minor T allele and the genotype TT are associated with depression (MDD, mixed anxiety depression, and dysthymia) among men [40]. Several studies reported the significance of this gene polymorphism in the risk of MDD, gene-environment interactions in depressive disorders, and treatment outcome of depression. These results suggest the association between dysthymia and this gene. Their involvement in the pathogenesis, risk and (or) treatment outcomes must be elucidated by future studies. Table 1 gives information on dysthymia or PDD associated SNPs reported by several studies.
Table 1: SNPs associated with Persistent Depressive Disorder.
|
Gene |
Reference |
SNP/Haplotype |
OR |
P-value |
Risk allele |
Sample origin |
|
CRY2
|
Kovanen et al., 201329
|
rs10838524 |
1.75 |
0.00002 |
G |
Sweden |
|
rs7121611 |
1.74 |
0.00002 |
A |
|||
|
rs7945565 |
1.71 |
0.00003 |
G |
|||
|
rs1401419 |
1.71 |
0.00004 |
G |
|||
|
Kovanen et al., 201730 |
rs10838524 |
1.56 |
0.00009 |
G |
Sweden |
|
|
rs7945565 |
1.59 |
0.00004 |
G |
|||
|
rs1401419 |
1.59 |
0.00004 |
G |
|||
|
rs7121611 |
1.59 |
0.00004 |
A |
|||
|
rs3824872 |
0.61 |
0.001 |
T |
|||
|
BDNF |
C.Lavebratt et al., 201044 |
rs6265 |
1.3 |
0.01 |
A |
Sweden |
|
IL-1
|
Fertuzinhos et al., 200450
|
IL-1RA (allele 2+)/ IL-1α (allele T+)/ IL-1β (allele C+) |
- |
0.002
|
- |
Brazil |
|
IL-1RA1.2/IL-1α C/T/ IL-β C/C |
- |
0.012 |
- |
|||
|
PRKCDBP |
Kovanen et al., 201730 |
rs1488864 |
1.58 |
0.0002 |
A |
Sweden |
|
FKBP5 |
C.Lavebratt et al., 201044 |
rs1360780 |
1.7 |
0.04 |
T |
Sweden |
GENE-ENVIRONMENT INTERACTIONS
The gene-environment interactions are observed when one or more gene variant(s) along with environmental factors make an impact on the phenotype [53]. Genetic factors along with environmental factors contribute to human disease by conferring vulnerability, and if the disease occurs by influencing severity and progression, the genetic analysis should prove as a powerful tool for researching mental disorders. Environmental factors were found to contribute to depression vulnerability, such as exposure to several infections, deficiency of nutrients, affectionate stress, societal disadvantage, racial minority, childhood maltreatment, use of cannabis, and stress [54–58]. Childhood abuse and maltreatment has been stated that the most imperative factors related with mood disorders such as bipolar and depressive disorders [59]. Several studies found the GxE relationship in depression, genes including BDNF, HTR2A, MAOA, SLC64A are related to several environmental factors such as childhood maltreatment, childhood adversity, and stress [53]. It was also observed that the adversities experienced during childhood result in changes in the brain structures, in turn, leading to depression [59]. The exact role of 5-HTTLPR in an increased risk of psychological disorders in adults with history of childhood problems is questionable as researches produce contradicting results [39]. The possible interlink between MAOA, childhood problems and antisocial behaviors was studied and as same as the former, studies on this produced contrasting results [60,61]. A study in 2012 stated the increased vulnerability of DD in women with a history of problematic childhood. Studies which relate a genetic and abnormal neurogenesis research on persistent depressive disorder has consistently lagged behind that the research on major depressive disorder, and should be high-priority. As they described, many factors appear to contribute to persistent depressive disorder, including slower cognitive development, interpersonal, personality factors, emotional, environmental, and certain biological factors [62]. The interaction of childhood maltreatment and abusive events with genes in dysthymia was observed in various studies and is depicted in table 2.
Table 2: Gene-Environment interactions reported in PDD or DD.
|
Gene |
Environmental factors |
GxE* |
Reference |
|
SLC64A |
Childhood maltreatment
Childhood maltreatment, Sexual abuse |
Individuals experiencing childhood maltreatment with the occurrence of short alleles of 5-HTTLPR(5Hydroxytryptamine transporter-linked polymorphic region) are at risk of PDD. The study did not determine the involvement of persistent depression in the chronic and recurrent depressive episodes, yet support the reported interactions between 5-HTTLPR polymorphism and childhood maltreatment in persistent depression. |
Brown et al., 2013
Helen et al.,2013 |
|
MAOA
|
Childhood physical abuse, multiple maltreatment (females) |
The homozygous high-activity allele 4, 4 of MAOA increases the vulnerability to DD in women with a history of physical abuse and maltreatment. |
Nikulina et al., 201235 |
|
BDNF |
More than two childhood problems |
A suggestive association of the Met-allele of BDNF (Val66Met) with Depression (MDD, mixed anxiety depression and dysthymia) in women with childhood problems. |
Lavebratt et al., 201044 |
CONCLUSION
This study has highlighted that psychiatric disorders are considered important in the case of disability universally. Identification of candidate genes is difficult because psychiatric illnesses are multi-genic, undefined connection between genetic and non-genetic or environmental factors, and have difficulty in phenotypic identification. This applies to the case of PDD. Many studies have already reported various genetic aspects of PDD, yet, there are several limitations. Additionally the genetic aspect, there are numerous factors such as isolation, trauma, and loss of close ones are also linked with PDD. Cross-Disorder work Group published article in the year 2013 study focused on the many highly GWAS study of psychiatric disorders, exposure that precise single-nucleotide polymorphisms study and range of associated of infancy or adult-onset psychiatric disorders [63]. The complication begins when studies target to determine the effect of genes in susceptibility of PDD with environmental factors. Amongst all the environmental factors, the interactions between PDD susceptible genetic polymorphisms and childhood trauma were reported in many studies and should be enquired in future research. Although several studies have found in PDD over the years and genetic predispositions were reported, the reliability of the studies should be elucidated by future investigations. Therefore, future studies should aim at identifying the heritability of PDD in various populations to focus on molecular and association studies have helped to establish the genetic and environmental basis of disease in particularly related to genes and depression level identified more specifically.
ACKNOWLEDGMENT
Authors thank Chettinad Academy of Research and Education (CARE) for the constant support and encouragement.
FUNDING
There is no funding in this research.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 62(6):617–627.
- Kang HJ, Kim SY, Bae KY, Kim SW, Shin IS, et al. (2015). Comorbidity of depression with physical disorders: research and clinical implications. Chonnam medical journal. 51(1):8–18.
- Winkler P, Horáček J, Weissová A, Šustr M, Brunovský M. (2015). Physical Comorbidities in Depression Co-Occurring with Anxiety: A Cross Sectional Study in the Czech Primary Care System. International journal of environmental research and public health. 12(12):15728–15738.
- Licht-Strunk E, van der Windt DA, van Marwijk HW, de Haan M, Beekman AT. (2007). The prognosis of depression in older patients in general practice and the community. A systematic review. Family practice. 24(2):168–180.
- Diagnostic and Statistical Manual of Mental Disorders (4th Edition) (2000). Text Revision. American Psychiatric Association, Washington, DC, USA.
- Sansone RA, Sansone LA. (2009). Dysthymic disorder: forlorn and overlooked?. Psychiatry (Edgmont (Pa.: Township)). 6(5):46–51.
- Rhebergen D, Graham R. (2014). The re-labelling of dysthymic disorder to persistent depressive disorder in DSM-5: old wine in new bottles?. Curr Opin Psychiatry. 27(1):27-31.
- Keller MB, Shapiro RW. (1982). "Double depression": superimposition of acute depressive episodes on chronic depressive disorders. The American journal of psychiatry. 139(4):438–442.
- Weissman MM, Leaf PJ, Bruce ML, Florio L. (1988). The epidemiology of dysthymia in five communities: rates, risks, comorbidity, and treatment. The American journal of psychiatry. 145(7):815–819.
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, et al. (2013). Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS medicin e.10(11):e1001547.
- Ballenger JC. (1999). Clinical guidelines for establishing remission in patients with depression and anxiety. The Journal of clinical psychiatry. 60 Suppl 22:29–34.
- Klein DN, Shankman SA, Rose S. (2006). Ten-year prospective follow-up study of the naturalistic course of dysthymic disorder and double depression. The American journal of psychiatry. 163(5):872–880.
- Cuijpers P, van Straten A, Schuurmans J, van Oppen P, Hollon SD, et al. (2010). Psychotherapy for chronic major depression and dysthymia: a meta-analysis. Clinical psychology review. 30(1):51–62.
- Klein DN, Clark DC, Dansky L, Margolis ET. (1988). Dysthymia in the offspring of parents with primary unipolar affective disorder. Journal of Abnormal Psychology. 97(3):265–274.
- Klein DN, Santiago NJ. (2003). Dysthymia and chronic depression: introduction, classification, risk factors, and course. Journal of clinical psychology. 59(8):807–816.
- Markkula N, Marola N, Nieminen T, Koskinen S, Saarni SI, et al. (2017). Predictors of new-onset depressive disorders - Results from the longitudinal Finnish Health 2011 Study. Journal of affective disorders. 208:255–264.
- Harkness KL, Wildes JE. (2002). Childhood adversity and anxiety versus dysthymia co-morbidity in major depression. Psychological medicine. 32(7):1239–1249.
- Griffiths J, Ravindran AV, Merali Z, Anisman H. (2000). Dysthymia: a review of pharmacological and behavioral factors. Mol Psychiatry. 5(3):242-261.
- Plornin R. (1992) “Behavioral Genetics,” Genes, Brain, and Behavior, personal communication.
- Hyman SE. (1999). Introduction to the complex genetics of mental disorders. Biological psychiatry. 45(5):518–521.
- Barondes SH. (1999). An agenda for psychiatric genetics. Archives of general psychiatry. 56(6):549–552.
- Argill M, Altshuler D, Ireland J, Sklar P, Ardlie K, et al. (1999). Characterization of single-nucleotide polymorphisms in coding regions of human genes. Nature genetics. 22(3):231–238.
- Lander ES, Schork NJ. (1994). Genetic dissection of complex traits. Science (New York, N.Y.). 265(5181), 2037–2048.
- Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, et al. (2014). Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it?. Biological psychiatry. 76(7):510–512.
- Kronfeld-Schor N, Einat H. (2012). Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology. 62(1):101–114.
- Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang RP, et al. (1996). Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry. 35(44):13871–13877.
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, et al. (2010). Cryptochrome mediates circadian regulation of cAMPsignaling and hepatic gluconeogenesis. Nature medicine. 16(10):1152–1156.
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, et al. (2012). Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America, 109(31), 12662–12667.
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. (2008). cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science (New York, N.Y.). 320(5878):949–953.
- Park SK, Nguyen MD, Fischer A, Luke MP, Affar e, et al. (2005). Par-4 links dopamine signaling and depression. Cell. 122(2):275–287.
- Kovanen L, Kaunisto M, Donner K, Saarikoski ST, Partonen T. (2013). CRY2 genetic variants associate with dysthymia. PloS one. 8(8):e71450.
- Kovanen L, Donner K, Kaunisto M, Partonen T. (2017). PRKCDBP (CAVIN3) and CRY2 associate with major depressive disorder. Journal of affective disorders. 207:136–140.
- Keltikangas-Järvinen L, Salo J. (2009). Dopamine and serotonin systems modify environmental effects on human behavior: a review. Scandinavian journal of psychology. 50(6):574–582.
- Naoi M, Maruyama W, Shamoto-Nagai M. (2018). Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis. Journal of neural transmission (Vienna, Austria : 1996). 125(1):53–66.
- Cicchetti D, Rogosch FA, Sturge-Apple ML. (2007). Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and psychopathology. 19(4):1161–1180.
- Beach SR, Brody GH, Gunter TD, Packer H, Wernett P, et al. (2010). Child maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorder. Journal of family psychology: JFP: journal of the Division of Family Psychology of the American Psychological Association (Division 43). 24(1):12–20.
- Nikulina V, Widom CS, Brzustowicz LM. (2012). Child abuse and neglect, MAOA, and mental health outcomes: a prospective examination. Biological psychiatry. 71(4):350–357.
- Melas PA, Wei Y, Wong CC, Sjöholm LK, Åberg E, et al. (2013). Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. The international journal of neuropsychopharmacology. 16(7):1513–1528.
- Verhagen M, van der Meij A, Janzing JG, Arias-Vásquez A, Buitelaar JK, et al. (2009). Effect of the 5-HTTLPR polymorphism in the serotonin transporter gene on major depressive disorder and related comorbid disorders. Psychiatric genetics. 19(1):39–44.
- Nobile M, Cataldo MG, Giorda R, Battaglia M, Baschirotto C, et al. (2004). A case-control and family-based association study of the 5-HTTLPR in pediatric-onset depressive disorders. Biological psychiatry. 56(4):292–295.
- Oliveira JR, Carvalho DR, Pontual D, Gallindo RM, Sougey EB, et al. (2000). Analysis of the serotonin transporter polymorphism (5-HTTLPR) in Brazilian patients affected by dysthymia, major depression and bipolar disorder. Molecular psychiatry. 5(4):348–349.
- Fisher HL, Cohen-Woods S, Hosang GM, Korszun A, Owen M, et al. (2013). Interaction between specific forms of childhood maltreatment and the serotonin transporter gene (5-HTT) in recurrent depressive disorder. Journal of affective disorders. 145(1):136–141.
- Aydemir O, Deveci A, Taskin OE, Taneli F, Esen-Danaci A. (2007). Serum brain-derived neurotrophic factor level in dysthymia: a comparative study with major depressive disorder. Progress in neuro-psychopharmacology & biological psychiatry. 31(5):1023–1026.
- Yoshimura R, Umene-Nakano W, Hoshuyama T, Ikenouchi-Sugita A, Hori H, et al. (2010). Plasma levels of brain-derived neurotrophic factor and interleukin-6 in patients with dysthymic disorder: comparison with age- and sex-matched major depressed patients and healthy controls. Human psychopharmacology. 25(7-8):566–569.
- Kim JM, Stewart R, Kang HJ, Bae KY, Kim SW, et al. (2015). BDNF methylation and depressive disorder in acute coronary syndrome: The K-DEPACS and EsDEPACS studies. Psychoneuroendocrinology. 62:159–165.
- Lavebratt C, Aberg E, Sjöholm LK, Forsell Y. (2010). Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. Journal of affective disorders. 125(1-3):249–255.
- Ho PS, Yen CH, Chen CY, Huang SY, Liang CS. (2017). Changes in cytokine and chemokine expression distinguish dysthymic disorder from major depression and healthy controls. Psychiatry research. 248:20–27.
- Anisman H, Merali Z. (1999). Anhedonic and anxiogenic effects of cytokine exposure. Advances in experimental medicine and biology. 461:199–233.
- Schlatter J, Ortuño F, Cervera-Enguix S. (2001). Differences in interleukins’ patterns between dysthymia and major depression. European Psychiatry. 16(5):317-319.
- Anisman H, Ravindran AV, Griffiths J, Merali Z. (1999). Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Molecular psychiatry. 4(2):182–188.
- Brambilla F, Monteleone P, Maj M. (2004). Interleukin-1beta and tumor necrosis factor-alpha in children with major depressive disorder or dysthymia. Journal of affective disorders. 78(3):273–277.
- Fertuzinhos SM, Oliveira JR, Nishimura AL, Pontual D, Carvalho DR, et al. (2004). Analysis of IL-1alpha, IL-1beta, and IL-1RA [correction of IL-RA] polymorphisms in dysthymia. Journal of molecular neuroscience: MN. 22(3):251–256.
- Uher R. (2014). Gene-environment interactions in common mental disorders: an update and strategy for a genome-wide search. Social psychiatry and psychiatric epidemiology. 49(1):3–14.
- Lin YL, Wang S. (2014). Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behavioural brain research. 259:24–34.
- Gámez-Guadix M, Orue I, Smith PK, Calvete E. (2013). Longitudinal and reciprocal relations of cyberbullying with depression, substance use, and problematic internet use among adolescents. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 53(4):446–452.
- Gullander M, Hogh A, Hansen ÅM, Persson R, Rugulies R, et al. (2014). Exposure to workplace bullying and risk of depression. Journal of occupational and environmental medicine. 56(12):1258–1265.
- Cummings CM, Caporino NE, Kendall PC. (2014). Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological bulletin. 140(3):816–845.
- Feingold D, Weiser M, Rehm J, Lev-Ran S. (2015). The association between cannabis use and mood disorders: A longitudinal study. Journal of affective disorders. 172:211–218.
- Jaworska-Andryszewska P, Rybakowski JK. (2019). Childhood trauma in mood disorders: Neurobiological mechanisms and implications for treatment. Pharmacol Rep. 71(1):112-120.
- Byrd AL, Manuck SB. (2014). MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biological psychiatry. 75(1):9–17.
- Haberstick BC, Lessem JM, Hewitt JK, Smolen A, Hopfer CJ, et al. (2014). MAOA genotype, childhood maltreatment, and their interaction in the etiology of adult antisocial behaviors. Biological psychiatry. 75(1):25–30.
- Schramm E, Klein DN, Elsaesser M, Furukawa TA, Domschke K. (2020). Review of Dysthymia and Persistent Depressive Disorder: History, correlates, and clinical implications. Lancet Psychiatry. 7(9):801-12.
- Cross-Disorder Group of the Psychiatric Genomics Consortium, Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013. 381(9875):1371-9.