Previous Issues Volume 9, Issue 1 - 2024
Gastroprotective Potential of Indian Medicinal Plants- A Comprehensive Review
Suchandra Goswami (nee Banerjee)1,*, Sruti Karmakar2, Koushik Brahmachari3, Prashanta Sarkar4, Nisha Sharma5, Mousam Ghosh1, Sanchita Maji1, Susanta Koley1, Santanu Shaw1, Diptendu Goswami6
1Bengal School of Technology (a College of Pharmacy), India
2Department of Environmental Sciences, Asutosh College, Kolkata, India
3Bidhan Chandra Krishi Vishwavidyalaya, Mohanpur, Nadia, West Bengal, India
4J.B. Roy State Ayurvedic and Medical College, Kolkata, India
5Chhatrapati Shahu Ji Maharaj University, Kanpur, India
6Smt. Vidyawati College of Pharmacy, Gora Machhiya, Kanpur Road, Jhansi, India
*Corresponding Author: Suchandra Goswami, Ph D (Pharmacy), Bengal School of Technology (a College in Pharmacy); Sugandha More, Hooghly, West Bengal, India, Pin 712102; Email: [email protected]
Received Date: May 3, 2024
Published Date: June 3, 2024
Citation: Goswami S, et al. (2024). Gastroprotective Potential of Indian Medicinal Plants- A Comprehensive Review. Mathews J Gastroenterol Hepatol. 9(1):23.
Copyrights: Goswami S, et al. © (2024).
ABSTRACT
Due to its versatile active ingredients, Indian biodiversity provides ample opportunity to search for putative therapeutics for the worst needy health conditions. Medicinal plants offer many natural remedies and are recognized as safe therapeutics. In India, the majority of people suffer from gastrointestinal disease due to poor living conditions, abundant food, smoking, environmental pollution, etc. India is a rich biodiversity zone, and its natural resources, whether plant or mangrove, need to be reviewed. Plant families such as Fabaceae, Ephedraceae, Meliaceae, and Apocyanaceae are enriched in a number of bioactive ingredients that are worthy of drug profiling. Thirty-two plants from different medicinal families were chosen for the study of their gastroprotective properties. Disease, such as gastric disease, peptic ulcers, duodenal ulcers, inflammatory bowel disorders, regurgitation, heartburn, and gastric cancer, were explored. This review includes a thorough discussion on different antiulcer models, anti-Helicobacter pylori activity, anti-gastric cancer activity and clinical studies. The pharmacokinetic fate of promising plants has also been reviewed. A number of plants, namely, Alstonia scholaris, Terminalia arjuna, Terminalia macroptera, Terminalia chebula, Withania somnifera, Kaemferia galanga, Geranium Willfordii, Pelargonium sidoides, Saussurea lappa, Azadirachta indicaand Aloe vera, exhibited anti-H. pylori activity. The important bioactive constituents present in the extracts are coumarins, alkaloids, anthraquinone, terpenoids, glycolipids, glycoproteins, saponins, flavonoids, lignin, tannins, glycosides etc. Although thirty-two plants have been identified at different stages, new therapeutic remedies can be designed by studying the pharmacological effects of one or more extracts separately and in combination to generate combinations of active compounds.
Keywords: Anti-ulcer, Anti H pylori, Natural product, Pharmacology, Gastric, Clinical, Neem
INTRODUCTION
Several pathological manifestations complicate the normal functioning of the gastrointestinal tract. In the oesophagus and stomach, various diseases are known, such as heartburn, regurgitation, dysphagia, odynphagia, dyspepsia, nausea and vomiting, anorexia, gas and bloating, constipation, diarrhoea, acute and chronic abdominal pain, gastrointestinal (GI) bleeding, abdominal mass, GERD, motor disorders of the oesophagus and lower oesophageal sphincter, achalasia, hypergastrinemia, peptic ulcer disease, dyspepsia, upper GI bleeding, and H. pylori infection. The diseases known to occur in the stomach and duodenum are duodenal ulcers, gastritis, gastric polyps, adenocarcinoma, neuroendocrine tumors, and gastric cancer. Several bacteria as well as protozoa are involved in disease pathogenesis. The greatest concerns are Campylobacter jejuni-induced diarrhea, Clostridium perfringens, Bacillus cereus, Vibrio cholerae, E. coli, Vibrio parahaemolyticus, Shigella dysenteriae, Yersinia enterocolitica, Giardia lambia, and Amebiasis (Entamoeba histolytica). Several intestinal nematodes, such as the roundworm, hookworm, whipworm, pinworm, and Strongyloides stercoralis, play important roles in virulence. In the small intestine, the most common diseases are celiac disease (in the duodenum) and short bowel syndrome. Moreover, Crohn's disease, ulcerative colitis (affecting the colon), inflammatory bowel disease, Belantidium coli, Blastocystis hominis, Dientamoeba fragilis, and colorectal cancer are prevalent in the intestine [1-7]. In India, approximately 7.2% of the population suffers from inflammatory bowel syndrome (6% with constipation, 32% with diarrhea, and 42.4% mixed), 7.6% with GERD (gastroesophageal reflux disorder, 4.39/105 per year) from Crohn’s disease, and celiac disease is often reported with FGIDs (functional gastrointestinal disorders, 4.4%). Helicobacter pylori is mostly associated with peptic ulcer disease and duodenal ulcers (8:1) but not with gastric ulcers (30:1). H. pylori infection in children ranges from 22-57% (under age 5) to 80-90% in childhood. The maximum prevalence of gastric cancer reported by Mizoram has been 30%. Gastric cancer associated with Epstein–Barr virus accounts for 7–18% of cases. The increasing concern of side effects associated with conventional medicine (such as ulcer recurrence and antibiotic resistance) necessitates the search for alternative therapeutics, whether plant, microbial, biotechnological or immunologically derived.
Natural products have always been recognized as safe therapeutics. Of the new chemical entities belonging to the group of small molecules that were approved between 1981 and 2010, only 36% were of synthetic origin, while more than 50% were derived from natural products. Its origin has been identified as a higher plant. Clear-cut genetic and chemical analysis as well as morphological and anatomical characterization, taxonomy, and synonyms are prerequisites. Furthermore, the collection of plant material, accurate documentation, botanical identification, and preparation of voucher specimens are also challenging tasks. Species distribution is also an important point to study. Ecological and legal considerations are equally important in drug discovery regarding laws on plant access, the sharing of benefits and patentability issues with local governments in the countries of origin. The United Nations Convention on Biological Diversity (CBD) signed in 1992 focused on three points: conserving biodiversity, sustainably using genetic resources, and sharing benefits from their use fairly and equitably. A sustainable supply of herbal material is also a standardized point that has been achieved via the constitution of good agricultural and collection practices (GACPs). Furthermore, the Nagoya Protocol of 2011 explained the access to genetic resources and the fair and equitable sharing of benefits arising from the utilization of CBD to facilitate and smooth natural product-based drug discovery. In addition to accessibility, the quality of the plant material is also important. The chemical composition of the plant material depends on various factors, such as species identity, harvest time, soil composition, altitude, actual climate, processing and storage conditions, and the presence of endophytic organisms. Furthermore, the determination of specific molecular mechanisms and rigorous clinical trials for the approval of natural products are also challenging tasks [8].
Drug Discovery from Natural Products
Natural products often possess selective biological activity, target particular proteins, have great biological diversity and have advantageous ADMET (Absorption, distribution, metabolism, excretion, toxicity) characteristics. While considering drug discovery from medicinal plants, ethnopharmacological information about traditional use guides therapeutic efficacy in humans. Analysis of 122 plant-derived compounds revealed that 80% of the compounds possessed the same pharmacological activity as they did for their ethnomedicinal use. Ethnopharmacology involves the observation, description, and experimental investigation of traditionally used drugs and their bioactivities [8]. The essential steps in drug discovery and development are target identification, hit generation, hit-to-lead-to-candidate optimization, and preclinical and clinical evaluation of final drug candidates. During drug discovery, for structural simplification, step-by-step processes such as analysis of complex structures, determination of substructures (groups) important for biological activity, elucidation of the structure-activity relationships (SARs) and pharmacophores, and removal of complicating structural motifs are employed to generate structural analogs. Furthermore, fine-tuning is achieved by eliminating redundant chiral centers and reducing the number of rings. The activities of the molecules are retained by altering the key pharmacophores. A pharmacophore with its ensembled steric and electronic features ensures supramolecular interactions with a specific biological target structure and initiates (or blocks) its biological response. There is a risk of changes in pharmacological and toxicological profiles during analog design [9]. Natural products are structurally complex. They contain a much larger fraction of sp3-hybridized atoms at bridgeheads and more chiral centers. Structurally lower nitrogen contents and higher oxygen contents are found. Only 38% of the natural products containing aromatic rings were aromatic. According to the dictionary of natural products, 50% of structurally resolved products have no synthetic origin, and only 20% are found in marketed form [10].
Natural Products and Omics
Natural products (NP) have multiple origins, including plant, animal, marine organism, and microbial sources. The emergence of clinically relevant pathogens necessitates the discovery of antibiotics and warrants a search for novel bioactive metabolites in the field of microbial NPs. The advent of the genome mining approach has aided in genomics and metagenomics-based drug discovery in natural products. It detects and analyses the biosynthesis-related gene cluster (BGC), which provides key information for understanding and predicting a specific group of related metabolites. By identifying open reading frames (ORFs) in a gene sequence, the border of protein-encoding genes can be optimized, and subsequently, bioinformatic tools can help identify the protein sequence. Furthermore, molecular networking (MN: comprising mass spectrometry coupled with dereplication) coupled with genome mining has resulted in the generation of more information on BGC that can be useful in augmenting the discovery, isolation, and structural prediction of novel compounds generated by microbial strains [11].
Chemoinformatic methods have also played an important role in natural product drug discovery. This study has also allowed for profiling, prioritizing, and comparing the molecular descriptors and physicochemical and pharmacokinetic properties of a group of NPs. Further physicochemical parameters can be derived by simulating optimized conditions, and a better drug-receptor relationship can be explored. A quantitative estimate of druglikeness (QED) was developed by combining the physicochemical parameters. A score of 1 indicates high druggability, and a score of 0 indicates a compound with undesirable properties. In the hit-optimization process, structural optimization of the molecule can be performed by direct chemical modification of functional groups through isosteric replacement, addition, and alteration of the ring structure. Molecular design based on core structures, such as eliminating redundant chiral structures and scaffold hopping, can generate pharmacophore-oriented lead structures [12,13].
Activity-oriented view of different genera and families of natural products
Recent studies have also shown the need for a combination of phylogenetic, spatial, and physicochemical information, correlations between phylogenetic and biosynthetic pathways, and correlations between biodiversity and metabolite biodiversity to guide the selection of taxa for lead compound discovery. Most of the clinically approved antibiotics included nine antibiotic classes differentiated by the scaffold. According to a survey of Javanese seed plant species, the natural products with the most selective anti-infectivity, i.e., terpenes, phenylpropanoids, phenols, sugars, and lactones, are redundant in most of the active plants of biodiversity-rich regions. The fluorene motif in anti-infective metabolites has shown much potential. The classes of natural products with a high percentage of anti-infective metabolites are quinones (29%), xanthones (24%), anthracenes (23%), and coumarins (23%). The lowest proportion of anti-infective metabolites were sugar and glycosylated compounds (7%) [14]. There are specific families (approximately 17) that are drug-prolific; 80% of the approved drugs are believed to be concentrated in these families. This review investigated plants within this family only. The families are further characterized by the number of species (S), approved drugs (AD) and bioactive components (BC) –Streptomyceteceae (7556 S, 147 AD, 340BC) and Pseudonocardiaceae (747 S, 76 AD, 24 BC) in bacteria; Trichomonaceae (1990 S, 45 AD, 402 BC), genera Acremonium (57 S, 49 AD, 9 BC), and Emericellopsis (16 S, 5 AD, 3 BC) in fungi; Hominidae (great apes and humans)(16S, 243 AD, 270 BC);and metazoan superking with 59% approved drugs. In the Viridiplantae family (green plant), the important families are -Fabaceae (legumes) (5882 S, 36 AD, 968 BC), Ephedraceae (Mormon tea) (72 S, 19 AD, 51 BC), Papaveraceae (poppies) (215 S, 19 AD, 96 BC), Asteraceae (daisy) (7229 S, 17 AD, 1016 BC), Solanaceae (potato) (1223 S, 15 AD, 130 BC), Rubiaceae (2524 S, 15 AD, 218 BC), and Apocyanaceae (dogbane) (1401 S, 14 AD, 260 BC). Other metazoan families include Viperidae (venomous snake) (288 S, 18 AD, 7 BC) and Muridae (rodents) (532 S, 19 AD, 14 BC). Other important families are Herpervirideae (480 S, 15 AD, 12 BC), Brassicaceae (1999 S, 2 AD, 181 BC), Cornaceae (104 S, 60 AD, 60 BC), and Taxaceae (26 S, 4 AD, 12 BC) [15].
Elucidation of the characteristics of bioactive families of natural products:
Rubiaceae is the fourth largest family of angiosperms and includes approximately 12000 members with enormous ecological diversity, as well as morphological diversity, concerning life forms (perennials, annuals), growth forms (shrubs, vines, herbs), leaves and stipules, inflorescences, flower and fruit morphology, and corresponding changes in pollination and dispersal ecology. Rutaceae is a family with 2100 species distributed in 154 genera and is widespread in tropical and temperate regions of Australasia, America, and South Africa. A wide number of species are used in folk medicine. The family Rutaceae is important because it provides natural and bioactive compounds such as terpenoids, flavonoids, and coumarins. Coumarins have been extensively reported to be involved in cancer, inflammation, infectious disease, and the treatment of endocrine and gastrointestinal conditions [16]. The family Apocynaceae is the largest and most important family of angiosperms. Most plants are consumed as food by tribal people, whereas few are used as poisons. Alkaloids, terpenoids, steroids, flavonoids, glycosides, simple phenols, lactones and hydrocarbons, sterols, lignans, and sugars are most prevalent. The most important activities include antioxidant, anti-inflammatory, antimicrobial, and cytotoxic effects. Holerhenna antidysenterica and Rauwolfia serpentine are important plants [17] Members of the Apocynaceae (dogbane) family are highly important for the treatment of nosocomial infections. They are extensively used as antimicrobial and resistance-modifying agents. The root drugs used in this study are from the Indian Systems of Medicine. The Apocynaceae family is considered one of the largest and most economically important angiosperms. They occur as trees, shrubs, and vines. Plants are characterized by the presence of latex and metabolites, such as alkaloids, triterpenoids, flavonoids, steroids, phenols, lactones, and glycosides [18]. The species of the families include colorful flowers and opposite leaves. They are useful for the treatment of fever, malaria, gastrointestinal ailments, diabetes, and pain. Catharanthus roseus and Rauwolfia serpentine contain bioactive indole alkaloids as well as terpene indole alkaloids [19]. Spiroflavonoids have been described in more than 40 species of eight families, including Asparagaceae, Cistaceae, Cuprassaceae, Fabaceae, Pentaphyllacaceae, Pinaceae, Thymelacaceae, and Vitaceae [20]. The Meliaceae family comprises timber tree plants that are used extensively against bacterial and fungal infections. Lamiaceae members are unique in having different aromatic-bearing plant taxa, all of which have wide applications in food and pharmaceuticals for the development of flavour and fragrance. The active constituents are volatiles, essential oils, terpenoids, phenolics, alkaloids, and flavonoids [21]. The Asteraceae family is the largest family of angiosperms and is enriched in phytochemicals such as diterpenoids, flavonoids, and polyphenols. These compounds have antitumour, antifungal, antibacterial, anti-inflammatory, antioxidant, etc. In terms of their floristic properties, the following families are used for local herbal formulation and medicine (rich in alkaloids and flavonoids): Asteraceae, Rosaceae, Lamiaceae, Malvaceae, Ranunculaceae, Poaceae, Solanaceae, Polygonaceae, Plantaginaceae and Brassicaceae. In India, the most common families of alien taxa are Asteraceae, Poaceae, Brassicaceae, Fabaceae, and Lamiaceae. Fabaceae species belong to the legume family and are capable of being symbiotic with nitrogen-fixing bacteria. They have high protein content. Fabaceae is the third-largest family of flowering plants and has three subfamilies: mimosoid legume, mimosoideae; papilionoid legume, papilionoideae; and caesalpinoid legume, caesalpinoideae. In the Fabaceae family, both grain legumes (peas, fabe beans, lentils, green peas, and chickpeas) and cereal legumes prevail. The active constituents are tannins, dyes, spices, fibre plants, ornamental plants, and medicinal plants such as liquorice [22]. The Euphorbiaceae family is large and diverse and has important medicinal value and potential for toxin production. They exhibit diverse biological activities, such as cytotoxic, antimalarial, antibacterial, antifungal, enzyme inhibitory, and immunosuppressive effects [23]. Many species contain milky juice (toxin). Several glycerophospholipids and glycolipids are reported to be contained within this family. The chemical constituents reported to be present are triterpenoids, glycosides, diterpenoids, flavonoids, and 4α-methyl steroids [24]. The Combretaceae family consists of large families of herbs, shrubs, and trees, the largest of which are Combretum and Terminalia. In terms of the active constituents, triterpenes, flavonoids, lignans, and nonprotein amino acids are present. They exhibit diverse pharmacological activities, such as antifungal, antibacterial, immune-stimulating, anticancer, spasmolytic, and gastrointestinal disorders. The broad-spectrum antibacterial activity of the combretaceae was found to correlate with the triterpenoid content [25]. Menispermaceae plants are useful for fruits, and drupe endocarps are diverse [26]. They constitute two major subfamilies: tinosporoideae and meninspermoideae [27]. The Solanaceae (nightshade family) is composed of approximately 2700 species and includes crops of significant economic, cultural, and scientific importance; examples include potato, tomato, pepper, eggplant, tobacco, and petunia. The active ingredients alcohol, phenylpropanoids, carotenoids, terpenoids, acyl sugars, capsaicinoids, steroidal glycoalkaloids, nicotine, and tropane alkaloids are fruity, stinky and sticky, spicy, bitter, additive, and deadly [28]. The family Zingiberaceae is important for its medicinal properties. India is the richest and most diverse region of Zingiberaceae, with 20 genera and more than 200 species. They are annual or perennial rhizomatous herbs. The important genera are Alpinia, Amomum, Curcuma, Elettaria, Hedychium, Kaempferia, and Zingiber. The main active constituents are volatile oils and oleoresins. Rhizomes and fruits are used as stimulants, tonics, aromatics, food, astringents, and diaphoresis juice. The Geraniaceae family is composed of 830 species in five genera (Erodium, Geranium, Monsonia, Pelargonium, and Monotypic California). All the genera have flowers that secrete nectar [29]. The family Bignoniaceae is composed of woody plants, trees, shrubs, and lianas distributed into eight clades (Bignoniaceae, Catalpeae, Coleae, Crescenteae, Jacarandeae, Oroxyleae, Tecomeae, and Tourrettieae). The main active constituents found are iridoids, which exhibit pharmacological activities such as anti-inflammatory, antimicrobial, antioxidant, and antitumour effects [30]. Betulaceae belongs to a small birch family with 160 deciduous trees and shrubs. The active constituent is betulin, a class of pentacyclic triterpenoids obtained from the outer bark. The active constituents exhibit cytotoxic, anti-inflammatory, anti-HIV, antidiabetic, antimicrobial and antiprotozoal activities [31].
Biodiversity hotspot:
The social, economic and environmental implications of biodiversity change constitute a platform for interdisciplinary research. Species loss can reduce the productivity and increase the stability of plant communities. Loss of biological diversity can also reduce multiple ecological functions and services, such as pest control, pollination and the breakdown of organic matter. It has also been found that temporal alterations in taxonomic diversity are not parallel to changes in functional biodiversity [32]. Various biodiversity parameters can be probed, including species representativeness, wilderness coverage, coverage of important areas of megafaunna, and regulating ecosystem services. All these parameters can be probed by two methods in general, species-based approaches and ecosystem-based approaches, and these two approaches at the local scale can together explain a wider range of conservation goals [33]. Food-web structural complexity, community dynamic stability and ecosystem functioning are three major topics for explaining changes in biodiversity. The loss of large species, which are resource species, may generate future food webs that are less spliced and are ordered by chains of interactions and compartments. This in turn will lead to secondary extinctions and strong trophic cascades. Size-structured ecological studies can particularly define the role of each species across communities and the causes and consequences of biodiversity loss [34] Furthermore, disturbances and varying environments have direct and indirect effects (as indicated by changes in species richness, community composition, and species traits) on biodiversity and ecosystem functioning. Additionally, the implementation of ecological theory that addresses species-average body mass, stoichiometry and the effects of environmental conditions such as temperature are important for understanding the relationships between biodiversity and ecosystem functioning. The natural changes in biodiversity arise due to independent responses of species groups across trophic levels to environmental changes and interdependent responses of the species groups to biodiversity at other levels, such as predator and prey groups that covary in density and diversity. Rainforest transformation and land use intensification can equally impact both biodiversity and related ecosystem functions and provides a window for stakeholders and decision makers about the trade-off between crop choices and the stability of biodiversity and ecosystem functions. Thirty-five biodiversity hotspots were studied in detail, of which two hotspots were discussed as relevant to the work domains of the authors [35].
Western Ghat and Biodiversity
The western ghats, which extend along the west coast of India, span an area of 180000 square kilometer and include a major portion of the western ghats and Sri Lanka hotspots, a biodiversity rice area (ranging from north to south across the states of Goa, Maharashtra, Karnataka, Tamil Nadu and Kerala). This region contains more than 30% of all plants, fish, herpetofaunna, birds, and mammalian species in India. It is rich in endemic species, large mammals, wildlife sanctuaries, tiger reserves and national parks. The region contains a wide range of medicinal plantsand important genetic resources, such as wild relatives of grains (rice, barley, Eleucene coracana), fruits (mano, gancinias, banana, jackfruits), and spices (black pepper, cinnamon, cardamom and nutmeg). The western part of the hill of the western Ghats Mountains has very dry climatic conditions. Four major types of forests are found in the Western Ghats: evergreen, semievergreen (Maharashtra, Goa, Karnataka), moist deciduous and dry deciduous (Karnataka, Tamil Nadu). The largest areas covered moist deciduous forests (southern parts of Kerala and Karnataka, 66%), followed by semievergreen, dry deciduous, and finally evergreen forests (Wayanad evergreen forests, 80%). According to WWF2001, five regions of the Western Ghats exist: the northwestern ghat montane rainforests, the southern western ghat mountain rainforests, the northern western ghat moist deciduous forests, and the southern Deccan plateau dry deciduous forests. Approximately 4000 flowering plants have been reported in Western Ghats (1500 species are endemic). The Nilgiri Mountains are the most important points for flowering plants (82 species are restricted in this region); other zones are the Agasthyamalai-Nilgiri hills and the Sri Lankan highlands. Southern western Ghats are enriched in tree species, whereas northern Ghats are enriched in herb species. In terms of fauna, the hotspot was enriched in birds (508 species, 4%), fishes (218, 53%), reptiles (157, 62%), mammals (137, 12%), and amphibians (126, 78%). Butterflies (5 families) and ants are the insects found. The region enriched in butterflies covers Agasthyamalai to the Palghat gap. Furthermore, high levels of butterfly diversity are found in Goa and Uttar Kanada. A total of 58 protected areas (14 national parks and 44 wildlife sanctuaries) were found there. Both the Western Ghats and Andaman Nicobar islands are biogeographic zones. Forty-one protected areas are recorded to have tigers, leopards, elephants, gaurs, hornbills, wild dogs, Sambhars and King cobra. A total of 332 globally threatened species are present in the Western Ghats, 229 of which are plant species. Among the 332 species, 55 are critically endangered, 148 are endangered and 129 are vulnerable. The quality of the key biodiversity area covered a set of quantitative, globally consistent criteria: the regular outcome of significant numbers of one or more globally threatened species or restricted-range species. A total of 126 key biodiversity areas are found in the Western Ghats: 47 sites for amphibians, 24 for reptiles, and 64% for globally threatened species. Five landscape-scale corridor outcomes were defined based on the distribution of species outcomes, existing and potential forest connectivity, ranges of landscape species and topography and represented as (from south to north) Periyar-Agasthyamalai, Anamalai, Mysore-Nilgiri, Malnad-Kodagu, and Sahyadri-Konkan. The Anamalai corridor (Palni hills) ranks third in terms of the number of tree species endemic to evergreen and semievergreen forests. A large number of tree plantations are reported in the central and southern parts, covering the Valparai Plateau and the Munnar area. Coffee plantations are found inside the Malnad-Kodagu corridor. The Hill agrosystem is dominated by tea, coffee, rubber, oil palm, eucalyptus, cinchona, wattle and cloves [36].
Sundaland and Biodiversity
Sundaland is a large biogeographic continent region in southern Asia covering the area of Indochina (Cambodia, Laos, Vietnam, Thailand), the Thai-Malay Peninsula, Sumatra, Java, Borneo, and the Sunda self. SouthernThailand is located on the Thai-Malay Peninsula, which is bordered by the Gulf of Thailand to the east and the Andaman Sea to the west. This peninsula is rich in forest-grassland mosaic ecosystems or savannah corridors in the interior of Sundaland [36]. Phuket Island is located at the western edge of the Sundaland Block on a tectonic plate surrounded by three tectonic plates known as the Philippine Sea and the Australian and Indian plates.
The Sundaland hotspots span the western half of the Indonesian archipelago, with 17000 islands along the equator between Asia and Australia. The island is dominated by Borneo and Sumatra. Geographically, the hotspots cover high mountain ranges, volcanoes, alluvial plains, lakes, shrimps and shallow coastal water. The land covers six endemic bird areas, 15000 endemic plant species, 139 endemic bird species, 115 endemic mammalian species, 268 endemic reptile species and 280 endemic freshwater species [37]. Sundaland biodiversity includes seven legume families: Dipterocarpaceae, Ericaceae, Fagaceae, Lauraceae, Moraceae, Myristicaceae, and Sapindaceae. The legume contains a large proportion of herbs and lianas [38].
Andaman Biodiversity
The Andaman archipelago comprises 556 islands, islets and rocks and is covered with four large contiguous regions, namely, the North, Middle, Baratang, and South Andaman, which cover more than 5000 km2of the total area and are surrounded by many isolated islands. The forest types range from evergreen forests to deciduous forests and mangroves [39]. Medicinal plants used by the Karens of middle Andaman constitute plant species belonging to the Zingiberaceae and Fabaceae families, and plants with the highest values include Typha angustifolia, Millingtonia hortensis and Piper betle [40,41].
Mangrove, Sunderban and gangetic biodiversity
Mangroves are distinctive communities found in tropical and subtropical land‒sea lactones. Coral reefs are potential land builders that facilitate the formation of islands and the extension of seashores. They are woody plants that can tolerate the salinity of the open sea and form a zone by succession of species up to the upper tidal region. The prop roots of these plants penetrate deeply into anaerobic mud, bringing oxygen to deep water, and marine animals can attach to the generated surface. They also serve as a nursing ground for fishes and shrimp. Mangrove forests can also produce biomass levels suitable for timber purposes. A forest composed of trees and shrubs creates its ecosystem. The most adapted species are the following: arenchyma on all types of roots aboveground; leaf bases of Nypa fronds; lenticels along stems, propagules and roots; and viviparity (these species are mangrove species specific, and their families include Rhizophoraceae and Avicenniaceae) [38]. The Sunderban biosphere reserve is extremely rich in diverse aquatic and terrestrial flora and fauna.
Gastroprotective effect: mechanism of the in vitro model
Since ancient times, traditional folkhealers have routinely dispensed several parts of plants, either single or mixed, as oral therapeutics. In this review, we comprehensively document the gastroprotective potential of Indian medicinal plants. Few animal experimental models have been described. An ethanol-induced ulcer model is generated by reactive oxygen species, an increased inflammatory response, increased vascular permeability, increased infiltration of leucocytes and decreased cell proliferation. Pylorus ligation-induced ulcers are caused by increased gastric acid secretion and autodigestion of the gastric mucosa followed by breakdown of the gastric mucosal barrier. The main pathologies of indomethacin-induced ulcers are a reduction in surface hydrophobicity; the inhibition of the synthesis of prostaglandins; the inhibition of exogenous sulfhydryls and interleukin 1; increased acid secretion; increased pepsin activity; and increased free radical formation, leukotriene, and leukocyte activation. Furthermore, stress-induced ulcers are related to an increase in the formation of reactive oxygen metabolites, the inhibition of prostaglandin synthesis and the alteration of nitric oxide levels. Elevation or downregulation of pathological markers such as reactive oxygen species, myeloperoxidase, IL-1β, chemokine (C-X-C motif) ligand 2/ macrophage inflammatory protein 2 (CXCL2/MIP-2), nitric oxide, prostacyclin, prostaglandin E2, inhibition of lipoxygenase, xanthine oxidase, radical scavenging activity, a2-noradrenergic receptor, ATP-dependent K+ channel, glutathione, interleukin 10, epidermal growth factor, and vascular endothelial growth factor are critical hallmarks.
Gastroprotective effect of Indian Medicinal Plants
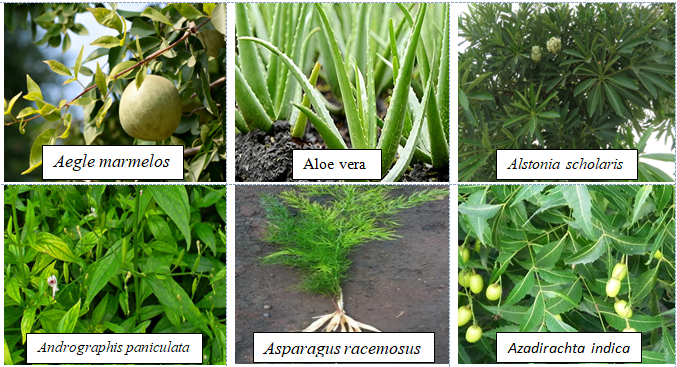
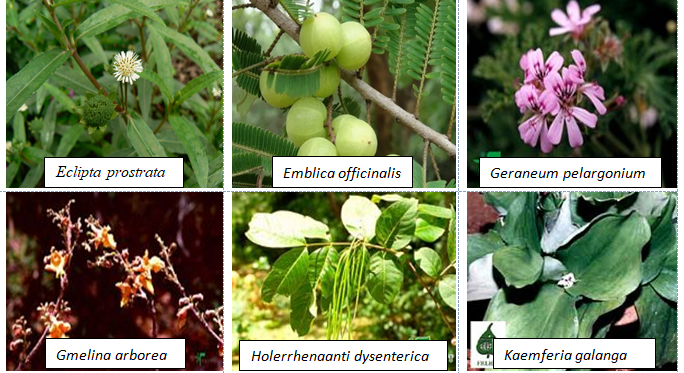
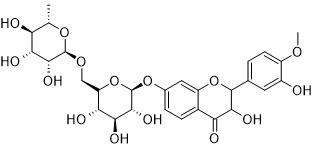
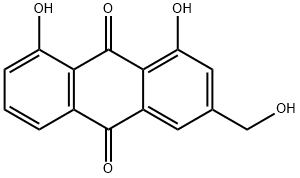
Among the 32 medicinal plants discussed throughout this review, six plants have been described in terms of their gastroprotective and pharmacokinetic properties: Aegle marmelos (Figure 6), Aloe vera (Figure 2), Andrographis paniculate (Figure 3), Ocimum sanctum (Figure 4), Phyllanthus emblica (Figure 5) and Azadirachta indica (Figure 1). The gastroprotective effects of the remaining plants are briefly discussed in this section. Alstonia scholaris is an active ingredient in the polyherbal formulation Ayush-64. This plant is reported to be rich in alkaloids, triterpenoids, sterols, isoflavonoids, and Proanthocyanidins. The important parts are flowers, bark and stem bark. It exhibited pharmacological activities such as anticancer, calcium channel blocking, urease inhibition, nitric oxide scavenging potential, anti-Helicobacter pylori activity (A. boonei), and ulcerative colitis activity (A. boonei) [42,43]. Asparagus racemosus has been reported to be active against gastric ulcers, galactagogues, functional dyspepsia, gastroduodenal ulcers, anti-candidal, anti-diarrheal, and apoptosis-inducing activities as well as immunomodulatory effects. It is rich in saponins, steroids, and shatavarin. It has also proven to be gastroprotective in clinical trials [44]. Bacopa monierii has been used as a gastroprotective agent. A literature study revealed a synergistic effect of A. indica on gastroprotection. It is rich in alkaloids, sterols, saponins, betulic acid and bacoside. Traditionally, it is used for stomach problems as well as constipation [45,46]. Bauhinia variegata leaf and flower extracts have been reported to exert dyspepsia, antimicrobial, antioxidant, anti-diarrheal, anti-ulcer, antisecretory, cytoprotective and anticancer effects. The seed contains galactose-specific lectins. The plant is rich in terpenes, flavonoids, steroids and alkaloids [47]. Betula utilis has traditional uses in the treatment of inflammation and cancer. The active constituents isolated from the bark are betulin, betulinic acid, lupeol, ursolic acid, oleanolic acid, and β-amyrin [48]. Catharanthus roseus has been reported in folklore medicine as a remedy for cancer, stomach ache. It is rich in monoterpene indole alkaloids (vincristine, vinblastine), bisindole alkaloids, flavonoids, and phenolic acid [49]. Clerodendrum serratum, Vitex negundo, and Terminalia chebula have all been shown to be potent against SARS-CoV-2. Clerodendrum serratum has been used in folklore medicine for the treatment of asthma and cough. It has been reported to have anti-inflammatory, antioxidant and anticancer effects. It is rich in saponin, flavonoids and phenolics [50]. Clerodendrum phlomidis has ayurvedic usage in the treatment of digestive disorders. It has shown anti-diarrheal activity. It is rich in chalcones [50,51]. Gum resin from Commifera wightii has traditional uses as an antiseptic, astringent, anti-ulcer, anthelmintic, carminative, emmenagogue, and expectorant agent. The active constituents are terpenoids and steroids. It possesses anticancer and anti-inflammatory activity. Commifera molmol has been proven to have gastroprotective activity [52]. Guggulsterone has shown potential in the treatment of inflammatory bowel disease [53]. Myrrh, an oleogum resin from Commifera molmol, has been shown to have a protective effect on barrier function in intestinal epithelial cell lines [54]. Guggulsterone (GS), a plant-derived steroid isolated from the gum resin of Commifora mukul tree, has shown anti-inflammatory and immunomodulatory effects. The combination of myrrh, chamomile flower and coffee charcoal has been proven to be effective for treating spasmolytic, anti-inflammatory and anti-inflammatory bowel diseases [55,56]. The essential oil of Cymbopogon winteriana (Citronella) possesses antioxidant, anti-inflammatory and antimicrobial activity. The active constituents are citral, α-elemol, geraniol, neral, and citronellal, which are present in the roots, root hairs with stalks, leaves and root silk [57]. Leaf extracts of Eclipta alba have shown promising antimicrobial, anticancer, and antioxidant effects. It has traditional use as a gastroprotective agent. It is rich in wedelolactone, luteon, and phenolics [58]. Eclipta prostrate has shown potential in therapeutic efficacy against inflammatory bowel disorder, osteoporosis, etc [59,60]. Gmelina arborea (GA) is traditionally used for preventing gastrointestinal disease [61]. GA stem bark has shown anti-ulcer activity. Gmelina Asiatica and Mallotus mudifordus were found to be adulterants. The nuclear ribosomal RNA-ITS1 (ribonucleic acid internal transcribed spacers) region was used as a molecular marker for authentic species. Iridoid glycosides are reported to be present in the aerial part of gambhari. The 4,8-dihydroxysesamine of Gmelina Arborea has shown potential against SARS-CoV-2 (severe-acute-respiratory-syndrome-related coronavirus 2). It has been reported to be effective at healing bone fractures and to have antimicrobial activity [61,62]. Holarrhena antidysenterica is widely used in traditional medicine for the treatment of intestinal peristalsis, constipation, colic, diahhroea, chronic amebiasis and antibacterial agents. It is rich in the alkaloid conesine [63,64]. In folklore medicine, Lawsonia innermis leaves soaked together with Psidium guajavaact in the treatment of stomach ache [65]. The active constituents of Lawsonia innermis leaves are naphthoquinone, terpene-β-ionone, volatile terpenes, single sterol, alkaloids, dioxin derivatives, lawsone, and luteon. Leaves have been shown to have a gastroprotective effect against gastric ulceration [66,67]. Extracts of lichen Parmelia Perlata have shown antiviral activity as well as antitoxin activity. Rauwolfia serpentina has been shown to exert anti-diahrroeal and anti-emetic effects. It is rich in the indole alkaloid reserpine. In DNA barcoding, two species-specific indels in rps16 (ribosomal protein S16) introns were found. Duodenal ulcers are a side effect when Rauwolfia is given clinically [68]. Saraca asoca contains high amounts of gallic acid, quarcetin (flower), and ellagic acid in its bark. Saraca asoca is traditionally used in folklore medicine to treat inflammation, stomach ache and dyspepsia. The plant is rich in flavonoids, tannins, sterol, leucocyanidin, quercetin, haematoxylin, and saraicin [50,69]. The gastroprotective and cytoprotective effects of Terminalia arjuna bark [70] have been reported. It has also shown potential in killing Helicobacter pylori. Triphala rasayana consists of fruits of three plant species, Phyllanthus emblica, Terminalia chebula and Terminalia bellerica, which are considered pillars for gastrointestinal treatment (constipation, gastric ulcers and inflammatory bowel disorders), especially for functional dyspepsia. Chebulinic acid isolated from the fruits of Terminalia chebula exhibited anti-secretory and cytoprotective effects. The roots of Terminalia macroptera and extracts of Terminalia chebula exhibited anti-Helicobacter pylori activity [71] and ROS inhibition. Terminalia chebula [72]. (Haritaki) thus has shown protective effects against H. pylori-induced gastrointestinal disease, peptic ulcers and gastric cancer. Trisama is a combination of Zinziber officinalis, Terminalia Chebula and Tinospora cordifolia, and reduced intestinal transit time [73]. Terminalia bellerica has also shown protective efficacy against gastric ulceration [50]. The tri-sa-maw recipe, a Thai recipe composed of Terminalia chebula, Terminalia sp. and Terminalia bellerica, is traditionally used to relieve tightness in the stomach and is a laxative and anti-diarrroeal agent [74]. Tinospora cordifolia (Guduchi, Giloy) has been used traditionally in Ayurvedic medicine because of its strong antioxidant activity, bitter tonics, and curative effects against diahhroea, dysentery, and helminths [75]. The plant has been found to be rich in diterpenoid lactone, glycoside, steroids, sesquiterpenoids, and phenolics in the stem, root and whole plant [76]. The roots of Vitex negundo have been used for the treatment of ulcers and colic pain [77]. The plant is rich in terpenoids, polyphenols, steroids, saponins, phenolic compounds and flavonoids. Monoolein has been isolated from the bark of Vitex negundo [78]. DNA barcoding of Vitex negundo was standardized using 42 ITS (internal transcribed spacer), psbA-trnH and matK (maturase K) gene accessions. Kaempferia galanga (Alpinia galanga) is traditionally used as an emmenagogue for chronic enteritis [79,81]. The plant is rich in essential oils, tannins, phenols, glycosides, monoterpenes, and carbohydrates [79,80]. It has shown protective effects against gastric ulcers. A phenylpropanoid derivative from the rhizome of Alpinia galanga exhibited gastroprotective activity. K. galanga extracts have shown the potential to kill H. pylori in an in vitro model. Geranium wilfordii is a traditional folklore medicine used to treat gastrointestinal disorders, diahhroea and dysentery. Geranium koreanum has shown putative gastroprotective efficacy and prevents esophageal damage in acute gastric reflux esophagitis [82]. Geranium thunbergii extract has shown potential for killing gastric cancer cells [83]. Geranium robertianum has also shown gastroprotective and antioxidant potential [84]. Geranium incanum has exhibited antidiarrhoeal activity. The rose scented geranium possesses essential oils. Nutmeg geranium essentially contains terpenoids [85]. Geranium wilfordii has also exhibited anti-Helicobacter pylori activity [86]. Geranium oil has shown antibacterial and antifungal potential. Geranium herb can antagonize the reduction in short-circuit current induced by cholera toxin, indicating its potential against toxin-induced secretion. EPs 7630, an extract of Pelargonium sodoides (rich in polymeric anthocyanidins), exhibited the potential to kill Helicobacter pylori. EPs 7630 can inhibit H. pylori. Pelargonium graveolens has traditional use in the treatment of gastrointestinal disease. The active constituents are volatile oils: citronellol, geraniol and citronellyl formate. Pelargonium reniforme has shown potential in the treatment of diarrhea. Mansoa alliacea (garlic vine, cipo aloh) has shown efficacy as an anti-inflammatory agent. The active constituents present are ρ-coumaric, ferulic and chlorogenic acids; pyranonaphthoquinones (manosonin A, B); luteolin; and apigenin [87]. Withania somnifera, as well as its active constituent withaferin A, has shown preventive potential against H. pylori and EBV-associated gastric cancer [88]. The extract has shown promising gastroprotective activity in an ulcer model of constipation and improved bowel function. The plant is rich in steroidal compounds, steroidal lactones, alkaloids, and amino acids. Saussurea lappa (Kushtha), a well-known endangered plant from Himalaya, contains terpenes, anthraquinones, alkaloids, and flavonoids. It has proven to have therapeutic potential as a gastroprotective agent both in vivo and in clinical trials. The main ingredients are costunolide, saussureanines, and dehydrocostus lactone [50]. The root has also shown potential as an antidiahhroea agent. It has also shown the potential to kill H. pylori in vitro. The plant is well known in the Indian Systems of Medicine. It has also shown anticancer activity in gastric AGS cell lines. S. lappa and its related species can be identified by the similarity (sequence) of the ITS-1, ITS-2 and 5S rRNAs. Saussurea pulchella has shown protective potential against ulcerative colitis (Table 1) [89].
Table 1: Morphology, active constituents, ethnopharmacological use and clinical trial of Indian Medicinal Plants.
|
Sl No |
Plant Name |
Family |
Ayurvedic name and Parts used* |
Active constituents |
Ethnopharmacological Use |
Clinical Use |
References |
|
Aegle marmelos L. |
Rutaceae |
Bilva; dried stem bark, leaves, pulp of unripe or half-ripe fruits, roots |
Coumarins and sterols; marmelosin, tannins, mucilage,fattyoil,sugar, auraptene,glycosides, Rutin, Aeglemarmelosine, Kaempferol-3-O- Rutinoside, Aegelinosides, Shahidine |
Diarrhoea, sprue, piles, oedema, jaundice, vomiting, obesity, deafness, eye diseases, paediatric diseases, fever and as a rejuvinative |
Radioprotective effect, Bronchial asthma, Type- 2 diabetes mellitus |
150,151 |
|
Aloe vera L. |
Liliaceae |
Kumari Ghritakumari; leaf pulp, dried leaf juice |
Anthraquinone glycoside; Aloe-emodin, Aloin/ Barbaloin, Aloesin, Emodin, Acemannan |
Splenomegaly, epilepsy, abscess, jaundice, abdominal lump, mastitis, headache and amenorrhoea |
Oral ulcer, teeth bone, Ulcerative colitis Seborrheic dermatitis |
125 |
|
Alstonia scholaris |
Apocyanaceae |
Saptapana; Dried stem bark |
alkaloids:Echitamine, ditamine, triterpenoids: Alstonic acid A, Alkaloids, triterpenoids, lignans, phenols |
Bronchial asthma, diabetes, ulcer, skin diseases, for purification of breast-milk, caries, poisoning and fever |
Bronchitis Eczema |
42,43 |
|
Andrographis paniculata |
Acanthaceae |
Bhunimba; Leaves and rhizome |
Andrographolide, 14-Deoxyandrographolide, 14-Deoxy-11,12-didehydroandrographolide, |
Worm infestation, skin diseases, jaundice, fever, acts as blood purifier. |
HIV, late stage cancer, COVID-19 Type-2 diabetes mellitus |
125,128,129,130,134 |
|
Asparagus racemosus |
Asparagaceae |
Shatavari; tuberous roots |
sugar, glycosides, saponin (Shatavarin I-IV), sitosterol, racemosides A, B and C Steroidal saponin,flavonoids, triterpene saponin,monoterpenoids |
Intrinsic haemorrhage, diarrhoea, piles, hoarseness of voice, cough, arthritis, poisoning, diseases of female genital tract, erysipelas, fever, as aphrodisiac and as rejuvinative |
Galactogogue, functional dyspepsia, Urinary tract infection |
44 |
|
Azadirachta indica |
Meliaceae |
Nimba; leaves, bark, flower, fruit, seed oil |
alkaloids, steroids, flavonoids, terpenoids, fatty acids, and carbohydrates tetranortritepenoids, azadirachtin, nimbolide, gedunin, nimbin, nimbidiol, nimbolicin, azadiradione, neem leaf glycoprotein, kaemferol, quercetin, myricetin, rutin, salanin, coniferyl alcohol and sinapyl alcohol, sulfonoquinovosyldiacylglyceride |
Fever, intrinsic haemorrhage, bleeding piles, wound, oedema, , arthritis, skin diseases, diabetes, eye diseases, leucorrhoea, as, poisons, jaundice, for fumigation, diseases of teeth, heart diseases, as specific digestive diseses |
Against Tinea corporis; plaque and gingivitis; periodontitis, Gastro-duodenal ulcer |
90, 92, 93, 98, 99, 101, 102, 103, 104, 105, 106, 107, 109 |
|
Bacopa monnieri |
Plantaginaceae |
Brahmi; whole plant |
Dammarane-type triterpenoid saponins: Jujubogenin, bacoside, bacopaside, bacopasaponin, alkaloids, glycosides, flavonoids, saponins |
Insanity, epilepsy, paediatric diseases, pox and as a rejuvinative |
Cognitive effect, anxiety, depression, alzeimers |
45,46 |
|
Bauhinia variegate |
Leguminocea; Fabaceae |
Kanchanara; dried stem bark |
Alkaloids, reducing sugars, terpenoids, glycosides, tannins, flavonoids, carbohydrates, bauhinone |
Worm infestation, skin diseases, goitre, leucorrhoea, cough, ulcer, emaciation |
|
47,50 |
|
Betula utilis |
Betulaceae |
Bhurjapatra; Stem bark, leaves |
Β-Amyrin, Lupeol, betulinic acid, Betulin, Ursolic acid, Oleanolic acid |
Jaundice, fever, ulcer, otitis media |
|
48,50 |
|
|
Catharanthus roseus |
Apocyanaceae |
Sadapushpa;root |
vindoline, vindolidine, vindolicine and vindolinine, vincristine, vinblastine, vindesine. |
Hypertension, Psychological disorder |
|
49,50 |
|
Commiphora wightii |
Burseraceae |
Guggulu; exudate |
Guggulsterone, Cembrene, gallic acid, quercetin |
Hyperlipidaemia, arthritis, diabetes, renal stone, rheumatoid arthritis, skin diseases, worm infestation, piles, goitre, ulcer, oedema |
Osteoarthritis |
52, 53, 57 |
|
Clerodendrum serratum |
Verbanaceae; Lamiaceae |
Bharangi / Agninrantha; root |
Terpenoids, Saponin, flavonoids, phenolics, Oleanolic acid, Queretaroic acid, Serratagenic acid, Ursolic acid, Lupeol, β- sitosterol, Spinasterol, Scutellarin |
Anaemia, oedema, cough, bronchial asthma, abdominal lump, fever |
Obesity |
50 |
|
|
Clerodendrum phlomidis |
Verbanaceae; Lamiaceae |
Arni; small stem |
Scutellarin |
inflammation, diabetes, nervous disorders, asthma, rheumatism, digestive disorders, and urinary disorders |
|
50, 51 |
|
Cymbopogon winterianes |
Gramineae |
Bhutrina; leaves, root |
Citronellal (37.4%), geraniol (23.6%) and citronellol (10.8%) – further main components (not presented): Elemol (3.9%) and limonene (3.4%) |
Eye diseases, mouth ulcer, constipation |
|
57 |
|
Eclipta alba |
Astaraceae |
Bhrngaraja; whole plant |
alkaloids, ecliptine, nicotine; Triterpenoid, saponin,alkaloids |
Filariasis, ulcer, wound, headache, giddiness, loss of vision, indigestion, enlargement of liver and spleen, jaundice, pain in the abdomen, cough, skin disease and fever. |
Diuretic, hypotensive, and hypocholesterolemic effects |
50 |
|
Eclipta prostrata |
Astaraceae |
Bhringaraja; aerial part |
Luteolin, Luteolin-7-O-b-D-glucoside,thiophene derivatives, steroids, triterpenes, flavonoids, polyacetylenes, polypeptides, coumestan, echinocystic acid, |
Infectious hepatitis, snake venom poison, gastritis |
|
58, 59 |
|
Emblica officinalis |
Euphorbiacea; phyllantheaceae |
Amalaki; fresh fruit palp, pericarp of dried, mature fruits, seeds |
Ascorbic acid, tannin, gallotannin; Phyllaemblic acid, β-Caryophyllene, β-Bourbonene Polyphenol,flavonoids, phenolicacid, flavones, alkaloids |
Fever, lack of appetite, piles, worms, anaemia, jaundice, intrinsic haemorrhage, hoarseness of voice, hiccup, fainting, heart diseases, vomiting, burning sensation, abdominal pain, leprosy, rheumatism, erysipelas, , pox, greying of hair, diabetes, suppression of urine, leucorrhoea, eye diseases, as preventive measure, for promoting intellect, as rejuvinative, and aphrodisiac |
Cardiovascular disease, hypertension, dyspepsia, Dyslipidemia |
141, 143 |
|
Gmelina arborea |
Verbanaceae; lamiaceae |
Gambhari; dried stem bark, dried mature root and root bark, fruits, stems |
alkaloids, flavonoids, lignans, iridoid glycosides, paulownin, gmelinol, and epieudesmin, Tyrosol (, balanophonin, gmelinol, phenylethanoid glycoside, |
Pain abdomen, burning sensation, fever, piles, emaciation, intrinsic bleeding, ulcer, retention of urine |
|
61, 62 |
|
Holarrhena antidysenterica |
Apocyanaceae |
Kutaja; dried stem bark Indrayava; dried seeds |
Conessin, holarrifine, kurchilidine, kurcholessine, kurchessine, flavonoids,treterpenoids, phenolic acid,tannin, resin, coumarin,saponin, ergosterol, regholarrhenin. |
Fever, diarrhoea, bleeding piles, leprosy and skin diseases, cough, calculi, gravels, wound and diabetes. diarrhoea |
Chronic ulcerative colitis, Amoebiasis |
63, 64 |
|
Kaemferia galanga (Aromatic ginger,Chandramooli) |
Zinziberaceae |
Chandramuli; rhizome |
δ-carene, 1,8-cineole |
Anti-swelling, headache, toothache, stomachache, rheumatism, antibacterial, antineoplastic |
Osteoarthritis |
79, 80, 81 |
|
|
Lawsonia inermis |
Lythraceae |
Madayantika; leaves |
Naphthoquinones (lawsone), coumarins, flavonoids, luteolin and its 7-O-glucoside, acacetin-7-O-glucoside; beta-sitosterol- 3-O-glucoside; all parts contain tannins. |
astringent, antihaemorrhagic, antispasmodic, oxytocic, antifertility, antifungal, antibacterial. Used externally to treat skin infections (tinea); also as a hair conditioner. |
|
65, 66 |
|
Ocimum sanctum |
Lamiaceae |
Tulasi; whole plant |
fixed oil and mucilages; essential oil(carvacrol, caryophyllene, Nerol, camphene etc); Eugenol, Eugenol methylether, Ursolic acid, Rosmarinic acid, Caffeic acid, Procatechuic acid, Gallic acid, Copaene, stigmasterol, Isorientin, Orientin, Apgenin, Luteolin Eugenol,β-elemene, β-caryophyllene, gallic acid, chlorogenic acid, essentialoil, fixed oil. |
Heart diseases, burning micturition, diseases of blood, skin diseases, cough with expectoration, asthma |
Gingivitis, oral submucous fibrosis, dental plaque, cognitive parameter, Peptic Ulcer diseases, COVID19 |
137, 138 |
|
Parmelia perlata (components of navratna oil) |
Lichens |
Saileya; whole thallus |
lichen acids like lecanoric acid, atranorin, usnic acid, phenolic compounds, terpenes,borneol, limonene,camphor, pulegone, thymol. |
Astringent, resolvent, diuretic. Used externally for pain in renal and lumbar regions. Also used as an ingredient in cosmetic preparations. |
|
50 |
|
Rauwolfia Serpentina |
Apocyanaceae |
Sarpagandha; dried roots |
Indole alkaloids such as reserpinine, serpentine, ajmalicine; calcium, phosphorus, sodium, potassium, magnesium, trace elements, Ascorbic acid, Riboflavin, Thiamine, Niacin |
Insomnia, to treat mental diseases, poisoning, dysentery, fever, ulcer, worm infestation. |
antihypertensive |
68 |
|
Saraca asoca |
Leguminoceae, Fabaceae, caesalphiniaceae |
Asoka; dried stem bark |
leucopelargonidin and leucocyanidin. Glycosides, lyoniside, nudiposide, 5-methoxy- 9-β- xylopyranosyl, isolariciresinol, and schizandriside, and three flavonoids, epicatechin, epiafzelechin-(4β→8)-epicatechin and procyanidin B2, together with β-sitosterolglucoside, Oleic, linoleic, palmitic and stearic acids, catechol, (-) epicatechol and leucocyanidin |
Menorrhagia, leucorrhoea, uterine disorders, worm infestation, burning sensation, emaciation, diseases of blood |
|
50, 69 |
|
Saussurea lappa |
Apocyanaceae |
Kushtha; Stem |
Dehydrocostus lactone, costunolide, cynaropicrin; terpenes: phellandrene, anethole; flavonoids, rutin |
Diabetes, menorrhagia, leukemia and stomach-ache |
|
50 |
|
Terminalia arjuna |
Combretaceae |
Arjuna; stem bark |
Arjunic acid, arjungenin, arjunetin; flavonoids: quercetin, kaempferol, luteolin, pellargonidin; tanins, glycosides. |
Heart diseases, consumption, spermaturea, skin diseases, fracture, intrinsic haemorrhage, wound, piles, diarrhoea. |
|
50, 74 |
|
Terminalia bellerica |
Combrataceae |
Bibhataka; pericarp of dried ripe fruits and seed |
Gallic acid, tannic acid, glycosides; chebulic acid, punicalagin, corilagin, chebulagic acid, casuarinin, bellaricanin |
cough, asthma, diarrhoea, oedema, vitiligo, eye diseases, erysepelas, calculi, heart diseases |
Anti bacterial activity salivary streptococcus mutant var., hyperlipidemia |
50, 74 |
|
Terminalia chebula |
Combretaceae |
Haritaki; pericarp of mature fruits |
tannin, anthraquinone, polyphonic compound; terchebulin, daucosterol, teaflavin A, chebupentol, terminoic acid |
Asthma, cough, diabetes, skin diseases, piles, oedema, hoarseness of voice, sprue, constipation, flatulence, hepatomegaly, splenomegaly, renal stone, burning micturition, retention of urine |
Hyperuricemia, anticaries, analgesic |
50, 72,74 |
|
Tinospora cordifolia |
Menispermaceae |
Guduchi; dried, mature pieces of stems, leaf |
terpenoids, alkaloids; Tinosporiside, Cordifolioside A, Cordioside, Furanolactone, Palmatine, Choline, Magnoflorine, Berberine, Beta-sitosterol Sinapic acid, Amritoside, Heptacosanol |
fever, irregular fever, chronic fever, jaundice thrist vomiting, arthritis, skin diseases, cough, as rejuveniative for purifying breast-milk, eye diseases |
Hypertriglyceridemea, Covid 19, scabies infection in paediatric, knee osteoarthriti, viral hepatitis
|
76 |
|
Vitex negundo |
Vernanaceae; Lamiaceae |
Nirgundi; leaf, seeds, root |
tritriacontane, n-pentatriacontane, nonacosane, β-sitosterol, Alkaloids (nishindine), Agnuside, Negundoside, Vitegnoside |
Abdominal pain, oedema, rheumatoid arthritis, worm infestation, skin diseases, dyspepsia, fever, eye diseases |
Cardioprotective, sciatica, |
78 |
|
Withania somnifera
|
Solanaceae |
Asvagandha; dried mature roots |
alkaloids, withanine, somnine, somniferine, Withanolide A, Withanone, Withaferin A, Withanolide Q, Ashwagandhanolide, , Sominone |
consumption, excessive emaciation, bronchial asthma, rheumatic ailments, insomnia, cardiac diseases, wound due to accident, suppression of urine, and for conception in sterility |
Stress reliever, muscle strength, Covid 19, schizophrenia, |
50, 88 |
*The traditional use of each plant has been obtained from the Ayurvedic Pharmacopoeia, envis kerala and Indian Medicinal Plants Glossary.
Meidicinal Plants
Pharmacology and Pharmacokinetics of Six Medically Important Indian Medicinal Plants
Azadirachta Indica
Approximately 50 publications have reported the gastroprotective effects of Azadirachta Indica. In one study, Azadirachta Indica (at doses of 100-800 mg/kg) p.o./i.p. significantly inhibited indomethacin-induced ulcer production (40 mg/kg) and exhibited 100% cytoprotection in albino rats. In another study, 500 mg/kg Azadirachta indica leaf extract exhibited antisecretory and proton pump inhibitory activities but did not affect mucus secretion. The extract was found to be safe up to a dose of 2.5 g/kg. Even at 1 mg/kg, no pathological alterations were observed. In another study, Azadirachta indica extract (250-1000 mg/kg) exhibited antiulcer and ulcer healing activity induced by physical (2 h cold restraint stress and 4h pylorus ligation) and chemical agents (ethanol, aspirin) as well as duodenal ulcers induced by cysteamine in normal and non-insulin-dependent diabetes mellitus (NIDDM) rats. Aqueous extracts dose-dependently inhibited gastric H+, K+-ATPase activity, oxidative membrane damage, and hydroxy (OH)-radical mediated mucosal DNA damage in vitro and protected against stress-induced apoptotic DNA fragmentation. Additionally, the extract exhibited more potent antiulcer activity than ranitidine in a stress ulcer model and greater acid antisecretory activity and was equipotent to ranitidine in a pylorus ligation model. In another study, neem extract (500 mg/kg dose) administered 12 hours after ethanol administration showed regenerative potential by restoring the ulcerated mucosal epithelium and reorganizing the cytoarchitecture [96]. In a clinical trial involving patients suffering from acid-related problems and gastroduodenal ulcers, 30 mg/kg aqueous neem leaf extract twice daily for 10 days caused a 69% decrease in gastric acid secretion and a 50% decrease in pepsin activity. Furthermore, doses of 30-60 mg twice daily for 10 weeks can completely heal duodenal ulcers, similar to esophageal ulcers and gastric ulcers [90]. In another study, the anti-Helicobacter pylori activity of neem oil extract was studied, and the MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) ranged from 25 to 51 µg/ml and from 43 to 68 µg/ml, respectively. The extract exhibited time- and concentration-dependent bactericidal activity and was found to be active at low pH [91-94]
In the benzo(α)pyrene-induced murine forstomach tumorigenesis bioassay protocol, aqueous Azadirachta Indica leaf extract at 100 mg/kg bw p.o. exhibited anticlastogenic activity, influencing oxidative stress and antioxidant defense.In another study, AAILE exhibited a decrease in phase I (cytochrome P450, cytochrome b(5), and aryl hydrocarbon hydroxylase) and an increase in phase II (glutathione-S-transferase and uridine 5'-diphospho-glucuronosyltransferase) activity as well as GSH (glutathione) content, indicating modulation of the peri-initiation phase of the process of forestomach tumorigenesis. Furthermore, another experiment showed that the extract increases benzo pyrene induced reduction of hemoglobin, red blood cells, and white blood cells; increased neutrophils; and modified eosinophils and basophils [95]. In another study, the ethanolic extract of Azadirachta indica leaf extract at 250 and 500 mg/kg body weight induced phaseII enzyme, hepatic glutathione-S-transferase (extrahepatic organ), and DT-diaphorse activity. It also elevates the levels of antioxidant enzymes such as hepatic glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase [96]. In another study, garlic and neem leaf extract exhibited potent anticarcinogenic activity in MNNG (methylnitronitrosoguanidine)-induced gastric carcinogenesis in male Wistar rats, accompanied by decreased lipid peroxidation, increased hepatic levels of glutathione and glutathione-dependent enzymes, and a protective effect at extrahepatic sites, altering hepatic biotransformation enzymes and antioxidants. Neem leaf extract alone at doses between 100-400 mg/kg body weight enhanced lipid peroxidation, even at a dose of 200 mg/kg, in MNNG-induced gastric carcinogenesis in rats. Another study reported that pretreatment with neem leaf extract significantly reduced MNNG-induced micronuclei and lipid peroxidases and enhanced GSH-dependent antioxidant activity. In addition, pretreatment with 100 mg/kg body weight attenuated the MNNG-induced increase in micronuclei and chromosomal aberrations. In another study, neem tree extract (NTE) at doses of 150, 300, and 600 mg kg body weight exhibited tumor regression activity in H-22 cell-bearing Kunming mice. Furthermore, the NTE can exhibit proliferative activity in immunogenic cells such as those in the thymus and spleen [97-99].
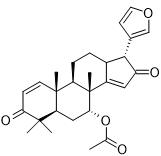
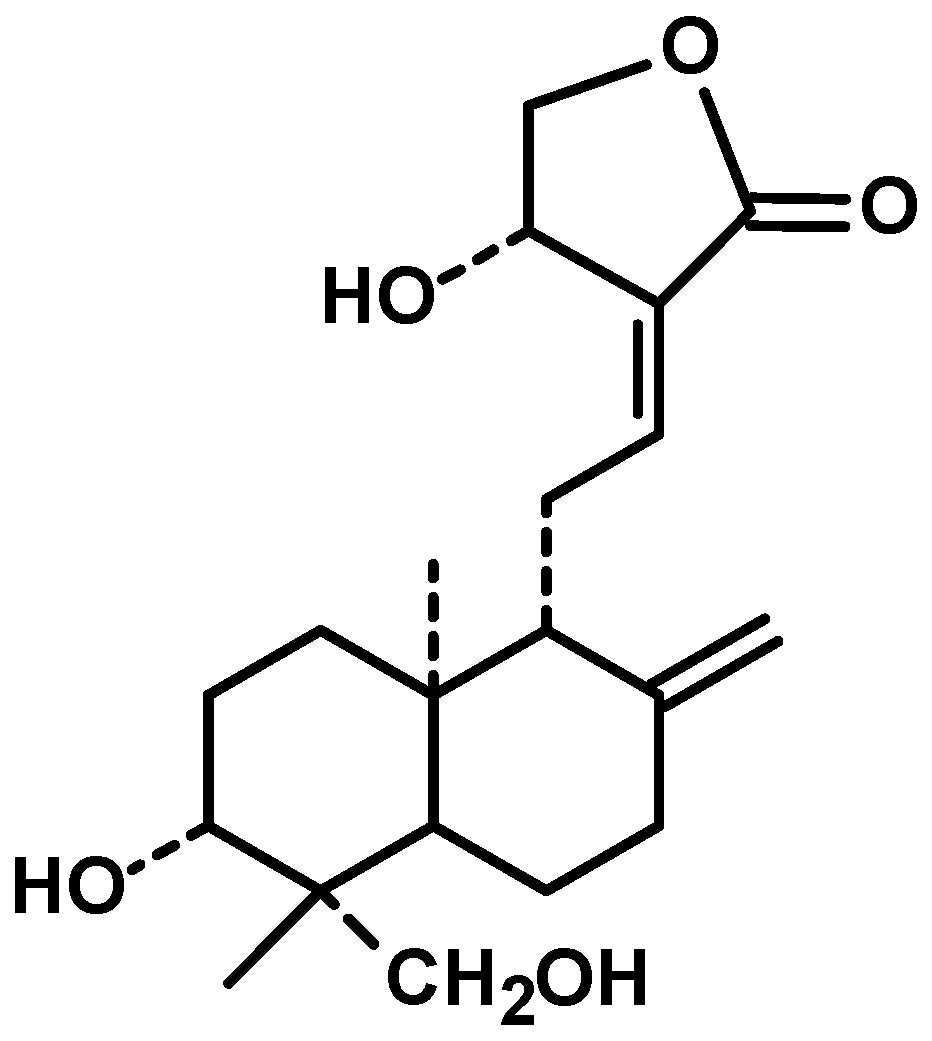
The pharmacological fates of a few active metabolites have also been reviewed. Azadiradione (Figure 1, 1), which is isolated from the ethanolic extract of Azadirachta Indica seeds, exhibited cytoprotective effects on a cold restraint-induced gastric ulcer model, an aspirin-induced gastric ulcer model, an alcohol-induced gastric ulcer model, and a pylorus ligation-induced gastric ulcer model and had significant inhibitory effects on gastric H+/K+-ATPase (hydrogen potassium adenosine triphosphate pump) [100]. Limonoids (Figure 1, 2) isolated from the bark, leaf, root, and seed extracts showed potent cytotoxicity against stomach cancer (AZ-521) cell lines. The compound exhibited apoptosis-inducing activity as well as activation of caspases 3, 8, and 9 with an increase in the ratio of Bax/Bcl-2 (B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) protein), indicating that both the mitochondrial and death receptor pathways are involved [101]. Tamarixetin 3-O-β-d-glucopyranoside (Figure 1, 3), which was isolated from the methanolic extract of neem leaves, exhibited gastroprotective activity against indomethacin-induced ulcers in mice. This compound also inhibits MMP-9 (matrix metalloproteinase) activity by binding to the pocket of the active site of MMP-9 and forming a coordination complex with a catalytic zinc [102]. Gadunin (Figure 1, 4) derived from neem trees effectively inhibited cell viability (IC50 of 20 µM), increased ROS generation, enhanced apoptosis in AGS (human gastric adenocarcinoma cell lines) cells, upregulated the bax gene, caspase 3, and caspase 9 and downregulated the bcl-2 gene [99,103-109].
|
Azadirachta indica |
Active constituents |
|
Azadiradione(1) |
|
|
Limonoides(2) |
|
|
Tamarixetin 3-O-β-d-glucopyranoside (3) |
|
|
Gadunin (4) |
Figure 1: Details of Azadirachta indica and its active constituents discussed.
Neem leaf extracts have also shown potential for potentiating the effects of other drugs. In one study, glipizide (5 mg/kg) administered concurrently with Azadirachta indica leaf extract (250 and 500 mg/kg) showed potentiating antidiabetic activity. Furthermore, leaf extract at 500 mg/kg augmented CyP3A (Cytochrome P450, family 3, subfamily A) activity, decreased the Cmax (maximum serum concentration), and increased the Tmax (time to peak drug concentration) to 8 h, indicating that leaf extract altered both the pharmacokinetic and pharmacodynamic profiles of glipizide. Another study reported the effect of an extract of MAMA decoction (MD) (an antimalarial product composed of leaves of Magnifera indica, Alstonia boonei, Morinda lucida and Azadirachta Indica) on enhancing the efficacy of amodiaquine (AQ) in malaria-infected mice. The dosing schedule was as follows: 3-tier, AQ (10 mg/kg/day) alone, second concurrently with MD (120 mg/kg/day), followed by MD pretreatment. The Cmax increased by 12% in the 2nd tier and 85% in the 3rd tier. Furthermore, the exposure and half-life of the major metabolite desethylamodiaquine increase by 11-21% and 20-33%, respectively [110]. Another study reported the effect of concurrent administration of A. indica on the pharmacokinetics of chloroquine sulfate. A significant decrease in serum concentration, slower absorption and elimination, and longer half-life of chloroquine were observed. There was a 71.9% decrease in the AUC, a 69.8% decrease in the Cmax, a 37.3% decrease in the absorption rate constant, a 53.9% decrease in the elimination rate constant, a 76.5% decrease in the clearance rate, and a 47.2% decrease in the volume of the distribution. A significant prolongation of the half-life of the drug (125.7%) was observed. A study of the pharmacokinetic effect of nimbolide in rats employing, LC/QTOF/MS (fast liquid chromatography quadrupole time-of-flight mass spectrometry) revealed poor absorption at doses between 10 and 50 mg/kg. Higher plasma concentrations were observed at an intravenous dose of 10 mg/kg [111].
Aloe vera (Aloe barbadensis)
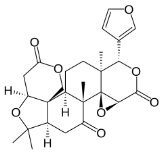
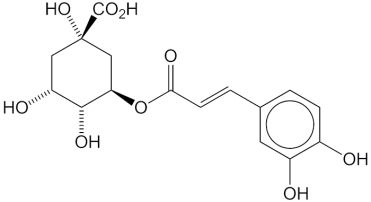
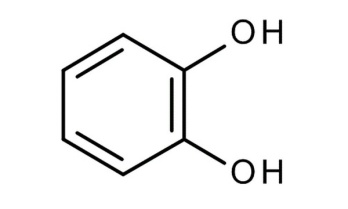
Aloe vera (150 mg/kg) in one study exhibited protective effects against indomethacin-induced gastric ulcers by decreasing the number of IL-18-positive cells, as well as reducing gastric erosion and neutrophil infiltration scores. In another study, an aloe vera pulp nanoemulsion inoculated with 2% activated yogurt culture of S. thermophillus and L. dellbreukii (1:1) exhibited protective effects against ethanol-induced gastric ulceration in rats. At doses between 10% and 30%, nanoemulsions cause a significant reduction in oxidative stress and ameliorate the release of different inflammatory mediators [MDA (malondialdehyde), NO (nitric oxide), catalase, NF-кB (nuclear fator kappa B), IL-1b (interleukin), MMP-9, homocysteine, ceramide] [112]. In a model of gastric ulcers induced by 20% acetic acid, Aloe vera protected against gastric ulcers by reducing leukocyte adherence in postcapillary venules and increasing the level of IL-10 but did not alter TNF-αlevels. Within 8 days, improvements in histological alterations (reduction in gastric inflammation, enhancement of epithelial cell proliferation, and elongation of gastric glands) were observed. In another study, Aloe vera (200 mg/kg dose, twice daily for 8 days) reduced duodenal water content as well as brain water content in a 20% acetic acid–induced model without having any preventive effect on gastric ulceration. In another study, the polymer fraction of aloe vera (150 mg/kg p.o.) significantly reduced ethanol-induced gastric ulceration in rats, with a 50% reduction in inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase, and MMP-9 levels in mice. The anti-H. pylori activity, an inhibition zone diameter between 1.6-1.65 cm was detected for two strains, with an MIC ranging from 15.62-3.9 µg/ml, and MBC ranging from 15.60-7.8 µg/ml. Aloe vera gel treated with chitosan nanoparticles increased DPPH (2,2-Diphenyl-1-picrylhydrazyl) scavenging activity and enhanced the percentage of haemolysis inhibition. The main components revealed by molecular modelling are chlorogenic acid (Figure 2, 5) and pyrocatechol (Figure 2, 6) [113]. In another study, processed aloe vera gel (PAG) significantly reduced the severity of intestinal ulcers and bacterial translocation and enhanced mucous secretion in indomethacin-induced ulcers in a mouse model. PAG was also reported to enhance mucin expression in the LS174T (adenocarcinoma associated colon cancer cell lines) human cell line via ERK (extracellular signal-regulated kinase) –dependent pathways. In another study, aloe vera inner gel leaf extract (a 5-year-old plant) at a b/w 800-1.50 mg/ml dose exhibited significant anti-Helicobacter pylori activity against 14 clinical (both drug-sensitive and drug-resistant) strains, and the bactericidal activity was found to be between 6.25 and 800 mg/ml [114]. In another study of the gastro-retentive NDDS (novel drug delivery system) of aloe vera, 100 mg of HPMC K15M (hydroxypropylmethyl cellulose), 27 mg of crospovidone, 80 mg of mannitol, and the use of effervescent agents at a 1:2 ratio resulted in 92% drug release; the combination of aloe vera gel and ellagic acid exhibited 75% ulcer protection compared to 57% with ellagic acid alone [115]. Another study reported the efficacy of aloe vera with amoxicillin in treating gastric ulcers with an optimized formulation of HPMC K4M and HPMC K100M (85:15 ratio) with a 1:4 ratio of effervescent agents that resulted in 97% release of drugs within 8h [116]. Another study reported that aloe perry leaf extract (APLE), flower extract (APFE), date palm seed extract (DPSE), and oily DPSE significantly inhibited ulcer induction and significantly reduced total and free acidity, the ulcer index, and gastric volume, with a masked decrease in gastric pH as well as mucosal strengthening [117]. Aloe vera gel was also evaluated against the sessile phase of clinical Helicobacter pylori strains (including multidrug-resistant strains) and against the planktonic and sessile phases of Staphylococcus aureus/Pseudomonas aeruginosa isolated from leg ulcers. Aloe vera exhibited antibiofilm activity with a decrease in biomass production in a concentration-dependent manner. The toxicological profile of nondecolorized aloe vera extracts was probed in rats (0.5%-1.5% dose) and in mice (1%-3%), eventually resulting in increased rates of hyperplasia in the large intestine, stomach, and mesenteric lymph nodes as well as the induction of cancer in the large intestine.118 In a diphenoxylate-induced constipation model, Aloe vera (75-300 mg/kg) reduced the discharge time of the first melena, improved the fecal conditions, increased the gastric intestinal charcoal transit ratio, and improved intestinal secretion in the small intestine [119]. In another study, aloe vera gel (50 and 300 mg/kg) exhibited protective effects on a 4% acetic acid-induced ulcerative colitis model in the rectum, resulting in reduced inflammation, ulcers, and tissue damage [120]. In another study, Aloe vera leaf pulp extract (30 µl and 60 µl/day/mouse daily for 14 days) reduced the levels of cytochrome P450 and cytochrome b5 (indicating that Aloe vera is a potent inducer of the phase II enzyme system). Furthermore, there was a decrease in malondialdehyde (MDA) formation and lactate dehydrogenase activity in the liver, indicating a protective effect against oxidant-induced membrane and cellular damage. Furthermore, the enhancement of microsomal and cytosolic proteins dictates the induction of protein synthesis. In extrahepatic organs (lung, kidney, and forestomach), the pulp extract exhibited activity against GST (glutathione-S-transferase), SOD (superoxide dismutase), and catalase. Thus, aloe vera could serve as a detoxifying reactive metabolite, including chemical carcinogens and drugs. Another study reported the efficacy of a mixture of Aloe vera (AV) and Matricaria Recuitta (50:50, at doses of 150-450 mg/kg) in treating IBS (inflammatory bowel syndrome) induced by 5 days of restraint stress in rats. German chamomile (GC) inhibited only small bowel transit, while the AV/GC mixture delayed gastric emptying (150-300 mg/kg) and reduced colonic transit (150/mg) and small bowel transit [121].
Figure 2: Details of Aloe vera and its active constituents discussed.
|
Aloe Vera |
Active constituent |
|
Chlorogenic acid (5) |
|
|
Pyrocatechol (6) |
|
|
Aloe- emodin(7) |
|
|
Emodin(8) |
|
|
Aloin(9) |
|
|
Aloesin(10) |
Aloe-emodin (Figure 2, 7), an anthraquinone glycoside isolated from the leaves of aloe vera, exhibited potent cytotoxic effects on the human gastric carcinoma cell lines AGS and NCI-N87 (gastric carcinoma cell line) by releasing apoptosis-inducing factor, cytochrome-c from mitochondria, and caspase 3 activation, resulting in nuclear shrinkage and apoptosis. Furthermore, it suppresses casein kinase II activity in a time-dependent manner, with reduced expression of the downstream regulator bid (BH-3 domain containing proapoptotic protein) and proapoptotic molecules. In another study, aloe-emodin arrested SGC-7901 cells in the G2/M (gap-2 phase/mitotic phase) phase and inhibited the expression of protein kinase C and c-myc (myelocytomatosis oncogene). In the MKN-45 human gastric cancer cell line, 0.05 mM aloe emodin (AE;figure 7) and emodin (EM; Figure 2, 8) significantly reduced proliferation (EM>AE); G0/G1 (quiescent phase/gap 1 phase) phase arrest was observed with AE, and G0/G1 and G2/M phase arrest was observed with EM, with significant differences in polyamine levels and DNA (deoxyribonucleic acid) fragmentation [122]. In another study, aloin (Figure 2, 9) isolated from aloe vera exhibited cytotoxicity in HGC-27 cells transfected with HMGB1 (high mobility group box 1). Cleaved PARP (Poly (ADP-ribose) polymerase), pro-caspase 3, HMGB1, and RAGE (receptor for advanced glycation end products) were found to significantly increase apoptotic activity. Aloin significantly reduced the rHMGB1-induced activation of the Akt-mTOR-P7OS6K (protein kinase B/ the mammalian target of rapamycin/ p70 S6 kinase) and ERK-CREB (extracellular signal-regulated kinase-cyclic adenosine monophosphate response element binding protein) signalling pathways [123].
In a clinical study, Aloe vera (10 ml) was administered thrice/daily to 240 patients with metastatic solid tumors treated with different chemotherapeutic agents (for lung cancer, cisplatin, etoposide, and vinorelbine; for colorectal cancer, oxaliplatin and 5-fluorouracil; for gastric cancer, 5-FU; and for pancreatic cancer, gemcitabine), and significant tumor regression and disease control were observed, as was the percentage of patients who survived for 3-4 years.
In one study, the effects of aloe, aloesin (Figure 2, 10), and propolis on the pharmacokinetics of benzo[α]-pyrene (BaP) and 3-OH-Bap in rats were investigated. Aloe influences the biliary excretion of BaP and urinary excretion of 3-OH-BaP, but only at higher doses (500 mg/kg). In another study investigating the efficacy of rhubarb peony decoction (RPD) (traditional Chinese medicine) and rhubarb extract per orally on the pharmacokinetics of aloe-emodin (Figure 2, 7), rhein, and emodin (Figure 2, 8) in rats, the Cmax of rhein in RPD decreased, the Tmax of rhein and emodin increased, and the elimination of aloe-emodin and emodin stopped. In another study investigating the bioavailability of Vit C (8 subjects) and Vit E (10 subjects) after 8h and 6h, respectively, a significant increase in the plasma concentration of ascorbate (500 mg ascorbic acid) and tocopherol (120 mg vitamin E acetate) was observed. Aloe could lower the absorption rate and prolong the presence of both vitamins in the blood. Two different aloe preparations at a dose of 2 oz (whole leaf extract and an inner filler gel) were used. In another randomized crossover trial, the modulation of the pharmacokinetics of vitamin C and vitamin B12 by two aloe vera extracts, aloe inner leaf gel (AG) and aloe whole leaf decoction gel (AL), was studied in human volunteers. In one group, 15 subjects received AG with vitamin B12 (1 mg) and vitamin C (500 mg), and the other 15 subjects received AL with vitamin B12 (1 mg) and vitamin C (500 mg) and were compared with control water (vitamin B12 (1 mg) and vitamin C (500 mg)). A study of the blood profile at 1,2,4,6,8, and 24h postfeeding revealed that AG increased the plasma oxygen radical absorbance capacity (ORAC) at both 4 and 24 h, as did AL for 4 h. The plasma concentration of vitamin C increased with AG at 4, 6, 8, and 24h and with AL at 4 and 6 h. Both formulations significantly increased the plasma VitB12 concentration at 1-2 h. In another study on the pharmacokinetics of aloe-emodin, rhein, emodin, chrysophanol, and their conjugates in rat plasma, the Cmax of aloe-emodin was much lower than that of rhein, and the Cmax of chrysophanol cum physion was below the detection limit. The metabolites were further traced upon treatment with β-glucuronidases. For aloe-emodin, rhein and emodin, the free/total ratios were 6.5 and 49, the Cmaxwas 1.7%, and the AUCs (Area-under the curve) were 3.7, 32.5 and 1.1, respectively. The Cmax values decreased in the following order: rhein>emodin>aloe-emodin>chrysophanol. The pharmacokinetic profile of Jinhongtang granules (JHTs), which are used for the treatment of severe infectious diseases, including COVID-19, was determined in normal and septic rats, and the Cmax and AUC of rhein, emodin, aloe-emodin, rhein-8-glucoside, aloe emodin-8-glucoside, protocatechuic acid, epicatechin and salidroside increased significantly in septic rats. In another pharmacokinetic study in rats, aloin A (barbaloin), the main bioactive anthraquinone-C-glycoside of Aloe species, was found to be quickly absorbed (absolute bioavailability 5.79%), extensively distributed, and rapidly eliminated [124,125].
Andrographis paniculata
In one study, the effects of aqueous (APLEE) and ethanolic (APLEE) leaf extracts of Andrographis paniculata (250 mg and 500 mg/kg b.w.) were studied in an ethanol-induced gastric ulcer model. A dose-dependent reduction in gastric ulceration and increased pH and mucin content were observed. The ethanolic extract exhibited overall better activity than the aqueous extract in reducing gastric ulcers as well as scavenging free radical-induced oxidation (ratio of IC50 10.9/24.65) [126]. In another study, the hydroalcoholic extract of Andrographis paniculata exhibited significant antiulcer activity at 200 mg/kg body weight (against ethanol, aspirin, pylorus, ligation, and cold restraint stress ulcer models). Significant reductions in the ulcer score, acidity, pepsin concentration, myeloperoxidase concentration, and H+/K+-ATPase activity were observed. Ethanol-induced increases in thiobarbituric acid reacting substance (TBASC) and decreases in glutathione and mucin levels are also therapeutically altered [127]. Another study revealed that Andrographis paniculata and andrographolide (Figure 3, 11) act as antistress agents by reducing corticosterone and cytokine homeostasis. In another study, the drug enzyme inhibitory effect onAndrographis paniculata (50 and 100 mg/kg b.w. for 14 days) was investigated. In the lung, kidney, and forestomach, significant increases in the levels of acid-soluble sulfhydryl, cytochrome P450, cytochrome P450 reductase, cytochrome b5 reductase, GST, DTD (DT-diaphorase), and SOD were detected. At higher doses, significant increases in the levels of catalase, glutathione peroxidase, and glutathione reductase were observed in the liver. A significant reduction in lactate dehydrogenase (LDH) activity and malonaldehyde formulation (MDP) was observed, indicating that Andrographis paniculata exhibited a significant effect against chemotoxicity-induced carcinogenesis. Andrographolide (Figure 3, 11) extract has also been shown to have an inhibitory effect on Epstein‒Barr virus (EBV), which is responsible for the induction of gastric cancer by suppressing the expression of EBV lytic proteins, completely abolishing the expression of the transcription factors MEF2D (myocyte enhancer factor 2D) and SP1 (specificity protein1), and inducing cell toxicity and apoptosis. EZH2 (Enhancer of zeste homolog 2) and HDAC6 (histone deacetylase 6), which are suppressors of EBV (epstein–barr virus) lytic reactivation, were significantly upregulated [128-131].
Figure 3: Details of Andrographis paniculata and its active constituents described in the text.
|
Andrographis paniculata |
Active ingredient |
|
andrographolide(11) |
|
|
14-deoxy-11,12-didehydroandrographolide (12) |
|
|
neoandrographolide (13) |
|
|
14-deoxyandrographolide (14) |
A liquid self-microemulsifying drug delivery system (SMEDDS) with 11.1% Andrographis paniculata extract, 40% capryol 90, 40% chromophore RH 40, and 8.9% Labrasol adsorbed onto colloidal silicon dioxide and microcrystalline cellulose followed by conversion in the SMEDDS by extrusion/sphereization exhibited 97% drug release within 15 min compared to that of the liquid extract, which released only 15%. In rabbits, the Cmaxvalues of andrographolide (Figure 3, 11) in liquid SMEDDS and SMEDDS pellets were 5-6-fold greater, and the AUC0-12h also increased 13-15-fold, indicating that the SMEDDS effectively improved the dissolution of Andrographis extract, which is otherwise poorly soluble.
The absolute bioavailability of andrographolide (Figure 3, 11) in MDCK (madin-darby canine kidney) cells overexpressing p-glycoprotein was found to be 2.67%. Permeability from the basolateral side was greater than that from the apical side. Furthermore, the most effective permeability was observed in the duodenum, followed by the jejunum, ileum, and colon. P-glycoprotein inhibitors such as verapamil significantly increase the permeability of andrographolide (Figure 3, 11) [132]. In another study, the LD50 (median lethal dose) of andrographolide (Figure 3, 11) was found to be 48 mg/kg bw (body weight), and the effective dose was 3 mg/kg. There was a significant reduction in the ulcer index (induced by ethanol, aspirin, or pylorus ligation), an increase in the pH, and a decrease in titratable acidity. It minimized the ulcer-induced increase in gastric H+/K+-ATPase and myeloperoxidase activity, indicating that the antiulcer effect of andrographolide (Figure 3, 11) is solely attributed to its antioxidant, cytoprotective and antacid secretory activity [133].
In another study, andrographolide (Figure 3, 11) inhibited the proliferation of the human gastric cancer cell lines SGC7901 and AGS, with IC50 values of 38 and 44 µM, respectively. It induces p53 protein expression, decreases mitochondrial membrane potential, inhibits cell proliferation, downregulates the levels of MDM-2 (mouse double minute 2 homolog), inhibits colony-forming ability, induces intrinsic mitochondrial apoptosis, such as caspase 9 and 3, cleaves PARP (Poly (ADP-ribose) polymerase), and inhibits BCL-2 (B-cell lymphoma 2) activity, indicating the use of andrographolide (Figures 3, 11) as a novel anticancer therapy. Andrographolide (Figures 3, 11) also exhibited anticancer activity in human endothelial cells expressing high levels of sialyllewis (X) by reducing E-selectin expression at the mRNA level and reducing cell adherence. In another study, andrographolide (Figure 3, 11) reduced clonogenicity in GC cells transferred with recombinant human tumor necrosis factor-related apoptosis-inducing ligand, causing induction of the TRAIL-R2 (death receptor 5) pathway, an increase in ROS (reactive oxygen species), and finally inducing apoptosis [134]. In one study, pretreatment with A. paniculata extract (APE) and its major component andrographolide (Figures 3, 11) (AG) for 3 days modulated the pharmacokinetics of theophylline, a cytochrome P4501A2 enzyme substrate, in rats (1-5 mg/kg). This result indicated an increase in clearance and a decrease in the area under the curve (AUC) in the pretreated animals. At high doses (5 mg/kg), the elimination half-life (t1/2β) and mean residence time (MRT) decreased to 14% and 17%, respectively. Theophylline accumulation was found in the APE-treated group, indicating the presence of other unidentified compounds in the APE extract, enhancing the therapeutic potential of theophylline. In another herb-drug interaction study, A. paniculata extract and andrographolide (Figure 3, 11) modulated the pharmacokinetics of glipizide in normal and diabetic rats, as evidenced by increased Cmax and AUC0-t/AUC0-α values. Furthermore, andrographolide (Figure 3, 11) could reduce various pharmacokinetic parameters in diabetic rats, as evidenced by the reductions in bioavailability, t1/2 values, Cmax, and AUC0-t/AUC0-α values. Another study investigated the therapeutic effects of four major active diterpenoids, namely, andrographolide (Figure 3, 11) (1), 14-deoxy-11,12-didehydroandrographolide (Figure 12) (2), neoandrographolide (Figure 3, 13) (3) and 14-deoxyandrographolide (Figure 3, 14) (4), found in Andrographis paniculata. In a pharmacokinetic study comprising 20 healthy volunteers (1:1 male to female ratio) 3 times, after dosing four capsules of 32.64, 5.40, 3.60, and 3.84 mg, the Cmaxvalues for compound 2 (44.89 ng/ml and AUC 128.17 h*ng/ml) were the highest, followed by compound 1 (Cmax 32.41 ng/ml and AUC 55.23 h*ng/ml). When compound 1 was used as the standard, the relative exposure to compound 2 was 14 times greater, that to compound 3 was 1.5 times greater, and that to compound 4 was 1.6 times greater. Furthermore, for compound 2, parameters such as Cmax, AUC, apparent volume of distribution, and apparent clearance significantly differed between the male and female groups. Another study investigated the effect of A. paniculata extract (2 g/kg/day APE) and andrographolide (Figure 3, 11) (50 mg/kg/day) on modulating the pharmacokinetic effect of tolbutamide in rats (for 5 days) and drug-metabolizing enzymes in the liver. The AUC0-12 h of tolbutamide decreased by 37% and 18% upon treatment with APE and AG, respectively. Significant increases in the mRNA and protein levels of CYP2C6/11, CYP1A1/2, and CYP3A1/2 were detected upon treatment. In high-fat obese mice, an increase in CYP2C6/11 expression was found in APE- and AG-treated rats, with a decrease in tolbutamide levels, indicating a potential accelerating effect of metabolism by APE and AG. However, there were no modulatory effects on antidiabetic activity. Another study investigated the pharmacokinetic and pharmacodynamic effects of A. paniculata extract (APE: 200 mg/kg) and andrographolide (Figure 3, 11) (AN:60 mg/kg) with the coadministration of naproxen (NP:7.5 mg/kg) in rats, and the systemic exposure level of NP was reduced. The remaining pharmacokinetic parameters, such as Cmax, Tmax, and AUC0-t, decreased. The modulation of etoricoxib (10 mg/kg) decreased the systemic Cmax, AUC, and t1/2 (half life) but increased the volume of distribution and clearance. Coadministration of APE synergistically increased its antiarthritic activity. Another study investigated the pharmacokinetics of A. paniculata extract and its metabolites andrographolide (Figure 3, 11), dehydroandrographolide (Figure 3, 11), and neoandrographolide (Figure 3, 13) in beagle dogs fed A. paniculata tablets (containing 49.5 mg of AP, 70 mg of DP, and 22 mg of NP). The t1/2 and AUC0-t (area under the curve up to the last quantifiable time-point) probed for AP, DP, and NP were found to be 2.08, 3.13, and 1.07 h –t1/2, respectively, and 494, 26.01, and 78.78 ng h/ml-AUC, respectively. In another study investigating the pharmacokinetic modulation of APE, AN on nabumetone in Wistar rats decreased the Cmax, AUC0-t, and AUC0-α (total drug exposure across time) of 6-MNA (6-methoxy-2-naphthyl acetic acid). With the AN Tmax increased to 2 h. With APE changes in mean residential time, the clearance and volume of distribution of 6-MNA were observed. Furthermore, with the AN + NAB combination, doubts about the presence of other interfering materials were raised. Furthermore, the significant decrease in antiarthritic activity indicates the negative impact of herb-drug interactions. Another study revealed that 20 mg/kg andrographolide (Figure 3, 11) (AND) was completely absorbed into the blood. Further bioavailability decreased by 4-fold at a 10-fold high dose. Fifty-five percent of the compound remains plasma bound. The pharmacokinetic profile can be described by one compartmental model in rats as well as two open compartmental models in humans. The Cmax in rats was 393 ng/ml (within 1.5–2 h), whereas in humans (3-4 tablets/day, approximately 1 mg AND/kg body weight/day), the value was 660 ng/ml. In another study investigating the effect of andrographolide (Figure 3, 11) (AP, a CYPA2 inhibitor) on the pharmacokinetics of aminophylline and doxofyllin in rats, the metabolism of theophylline (the active metabolite of doxophyllin) by AP was inhibited. Both combinations increased the AUC0-t value of theophylline. Andrographolide (Figure 3, 11) also has a slower renal clearance, indicating a negative impact of concurrent herb-drug interactions. In a single-center, randomized, single-dose, three-way crossover design, the pharmacokinetics of dehydroandrographolide (Figure 3, 11) succinate (DAS) were probed in healthy Chinese volunteers who received 80, 160, or 320 mg of DAS. The three doses resulted in Cmax values of 4.82, 12.85, and 26.90 mg/l and mean AUC0-∞ values of 126.8, 16.95, and 40.65 mg/L.h, respectively. DAS exhibited rapid clearance with a Tmax of approximately 0.94-1 h and t1/2 of 1.59-1.89 h. There was no sex-related variation in absorption. However, 10-15% of the i.v. DAS was excreted unchanged in urine within 24 h, and more than 90% of the unchanged DAS was excreted within 0-4 h, indicating nonlinear pharmacokinetic characteristics. By studying the bioavailability of the diterpenoid 14-deoxy-11,12-didehydroandrographolide (deAND) (Figure 3, 12) in rats (50 mg/kg dose), Cmax values of ~2.65 µg/ml, AUC0-24h values of 0.29 h, mean retention time of 6.30 µg/ml.h and a half-life of 3.56 h were found. Only 3.42% were bioavailable orally. Furthermore, at 10 µM, CYP4501A1/2, CYP2C6, CYP3A1/2, UDP-glucuronosyltransferase (UGT) 1A1, NAD(P)H (reduced form of nicotinamide adenine dinucleotide phosphate), quinone oxidoreductase (NQ01), the π form of GSH S transferase (GSTP), MDR2 (multi drug resistant 2), p-glycoprotein and organic anion transporter protein 2B1 were detected in rat hepatocytes, indicating the rapid passage of deAND in enterocytes and the upregulation of hepatic enzymes.
Ocimum Sanctum
In Ocimum sanctum leaves, Eugenol (Figure 4, 15) (1-hydroxy-2-methoxy-4-allylbenzene) is considered an active compound with multiple therapeutic potentials. The component has been extracted using steam distillation, petroleum ether, and benzene extracts from various plants of tulsi Fixed oil from Ocimum sanctum leaves has shown antiulcer activity against aspirin, indomethacin, alcohol, histamine, reserpine, serotonin, and stress-induced ulceration. Fixed oil exerted potential antisecretory activity in the pylorus ligation model. In another study, Ocimum sanctum extract significantly reduced the ulcer index and free and total activity upon acute and chronic treatment in pylorus ligation-induced and aspirin-treated ulcer models [135]. Another study reported the effectiveness of a methanolic extract of Ocimum sanctum (OSE:50-200 mg/kg, orally, twice daily) against cold restraint stress ulcers, alcohol-induced ulcers, and a pylorus ligation model. In 50% acetic acid-induced ulcers, 100 mg/kg OSE significantly prevented acid-pepsin secretion and lipid peroxidation. It also exhibited potential effects on mucus secretion, cellular mucus, the life span of mucus cells, and free radical scavenging activity. The extract has no potential to regenerate new mucus cells. Another study reported the antiulcer activity of O. Sanctum leaves at a 100 mg/kg dose, with 65.07% decreases in CRU, 63.49% in ASP, 53.87% in AL, 62.06% in pylorus ligation, and 61.76% in histamine-induced duodenal ulcers in rats. Complete healing of the ulcer was observed within 20 days. The extracts further exhibited significant cytoprotective and antisecretory activity by reducing free acidity by 72.58%, total acidity by 58.63%, peptic acidity by 57.6%, and mucin secretion by 34.61%. Another study reported the antioxidant activity of tulsi leaf extract (50 mg - 200 mg/kg orally once daily for 7 days) in an ethanol-induced ulcer model in rats and histamine-treated guinea pigs—a significant reduction in malondialdehyde (MDA) and superoxide dismutase activity (SOD) was observed between 100-200 mg/kg.
A subacute toxicity study of methanolic/aqueous extracts of O. sanctum in Wistar rats at doses of 250-1000 mg/kg/day for 28 days revealed no changes in body weight (wt), food or water consumption; motor or sensory activity; hematological, pathological, or biochemical parameters; or the urological or histopathology of tissues (liver, kidney, spleen, heart, testis/ovary). An OSE of 1000 mg/kg elevated the mean corpuscular haemoglobin (MCH) concentration and mean corpuscular haemoglobin concentration (MCHC).
In another study, the ethanolic extract of Ocimum sanctum (300 mg/kg 3 times per week) following the induction of gastric carcinogenesis with N-methyl-N-nitro-N-nitrosoguanidine (MNNG) for 15 days induced a significant reduction in gastric carcinogenesis, with consequently decreased expression of PCNA (proliferating cell nuclear antigen), GST-Pi (glutathione S-transferase pi gene), Bcl-2, cytokeratin, and VEGF (vascular endothelial growth factor) along with the overexpression of Bax, cytochrome c and caspase 3. Another study explored the anticancer activity of Ocimum tenuiflorum essential oil (OTEO) in a human gastric cancer cell line (AGS cells). OTEO exhibited apoptotic activity; induced cell shrinkage and chromatin condensation; inhibited cell migration and invasion in a dose-dependent manner; upregulated the expression of proapoptotic genes such as TP53 (tumor antigen p53), BAX (promote apoptosis), and BAK (Bcl-2 homologous antagonist/killer); downregulated the expression of antiapoptotic genes such as BCL-2 and BCCXL (B-cell lymphoma-2 inhibit apoptosis); and increased the gene expression of CASP8 (caspase), CASP9, and CASP3. The activity was found to be solely due to caryophyllene and α-pinene [136]. Another study evaluated the immunomodulatory effect of ethanolic extracts of tulsi leaves in a double-blinded randomized controlled crossover trial to assess the levels of Th1 and Th2 cytokines (interferon, interleukin-4), T-helper, T-cytotoxic, B cell, and NK (natural killer) cells. The levels of all the mediators decreased significantly after 4 weeks of treatment [137]. In another study, basil leaves exhibited potent anticarcinogenic activity in benzo-α-pyrene-induced squamous cell carcinoma in the stomach of Swiss mice and 3’-methyl-1-4-dimethylaminobenzene (3'-MeDAB)-induced hepatoma. Assessment of the combinatorial chemopreventive activity of Ocimum Sanctum (150 mg/kg) and Azadirachta indica (100 mg/kg) in N-methyl-N’-nitro-N-nitroguanidine (MNNG)-induced gastric carcinogenesis for 26 weeks revealed a decrease in carcinogenic events, with a decrease in PCNA, GST-P, CK (creatine kinase), VEGF(vascular endothelial growth factor), and Bcl-2 and upregulation of bax, cytochrome C and caspase 3, indicating inhibition of cell proliferation and angiogenesis and induction of apoptosis. In a randomized, parallel-group, open-level pilot study, O. sanctum extract (250 mg capsule, twice daily in an empty stomach for 8 weeks) improved the serum triglyceride, LDL (low density lipoprotein), HDL (high density lipoprotein), VLDL (very low density lipoprotein), BMI (body mass index), and plasma insulin levels. Furthermore, insulin resistance occurred. No effect on serum SGPT (Serum glutamic pyruvic transaminase) or SGOT (serum glutamic-oxaloacetic transaminase) levels was detected.
Eumil, an herbal formulation comprising extracts from Withania somnifera, Ocimum sanctum, Asparagus racemosus, and Embelica officinalis administered at a 100 mg/kg dose (once daily for 14 days p.o.), significantly inhibited gastric ulceration, and the effect was comparable to that of Panax ginseng. Furthermore, the formulation exhibited adaptogenic and antistress activity. Siotone (ST), a polyherbal formulation composed of Withania somnifera, Ocimum sanctum, Asparagus racemosa, Tribulus terrestris, and Shilajit, exhibited adaptogenic activity by altering the perturbation indices of chronic unpredictable foot shock stress-induced perturbations, such as behavior, glucose metabolism, immunosuppression, gastric ulceration and cognitive dysfunction, in albino rats. Furthermore, at doses between 50 and 100 mg/kg p.o., ST significantly improved gastric ulceration. In another study, the use of syferol-APP (a blend of virgin coconut oil and Ocimum sanctum oil) along with a pyloro test (a three-in-one tablet containing 20 mg rabeprazole, 1 g amoxicillin, and 500 mg clarithromycin) for 2 weeks resulted in the healing of peptic ulcer disease in 37 patients. Gastric ulcers (not duodenal ulcers) healed, with 100% eradication of H. pylori. Furthermore, a 16.2% reduction in epigastric pain and a 13.5% reduction in gastritis were observed [138]. The polyherbal formulation NR-ANX-C comprising extracts from Withina somnifera, Camellia sinensis, Ocimum sanctum, Shilajith, and Triphala at 25-50 mg/kg exhibited potent antiulcer activity (even better than that of ranitidine) in a pylorus ligation model. Furthermore, it induced a dose-dependent decrease in gastric juice volume and total activity and a dose-dependent increase in pH and total adherent gastric mucus, followed by a reduction in the extent of lipid peroxidation, making the formulation promising for the treatment of gastric ulcers. In another study, polyherbal formulations comprising Allium sativum, Eugenia jambolana, Momordiacharantia, Ocimum sanctum, and Psidium guajava exhibited increased hepatic and intestinal phase II enzyme levels; the activity of hepatic and intestinal UDP-glucuronyltransferase and glutathione-S-transferase increased with dose and time, whereas that of hepatic sulfotransferase increased only at the higher dose. Furthermore, CyP3A mRNA levels remain unaltered. Another study reported the modulatory activity of alcoholic extracts of tulsi leaf (400-800 mg/kg) on cytochrome P450, cytochrome b5, and aryl hydrocarbon hydroxylase in the liver and glutathione-S-transferase and reduced glutathione levels in the liver, lung and stomach in mice. A significant increase in the activity of all enzymes made the extract a promising detoxifier and mutagen. Furthermore, an increase in extrahepatic glutathione-S-transferase in extrahepatic cells was observed. In liver, lung, and stomach tissues, a significant increase in reduced glutathione levels was also observed. In another study, an emulsion-base composite from beeswax (1-3%) and carboxymethylcellulose were used to coat NDDSs on holy basil essential oil-loaded gelatine microcapsules (HBEDs) prepared by coacervation techniques. Only 2% beeswax-CMC-coated microcapsules exhibited sustained antioxidant activity; furthermore, the formulation allowed minimal interaction of the HBED in simulated gastric fluids with concurrent delivery of the oil to the distal small intestine.
In one study, while investigating the penetration-enhancing ability of tulsi and turpentine oil on the transdermal delivery of flurbiprofen, a potent nonsteroidal anti-inflammatory agent, the bioavailability of flurbiprofen was 3-8 times greater than that of tulsi. This finding was also confirmed by a pharmacokinetic assay. The neuroprotective and antiepileptic effects of Ocimum sanctum hydroalcoholic extract (OSHE) (200-1000 mg/kg orally for 14 days) were studied using maximal electroshock-induced seizures in a rat model. With valproate, there was no alteration in antiepileptic activity, but there was a significant enhancement in memory retention potential and protection against oxidative stress. Furthermore, the pharmacokinetic parameters also did not change significantly. In another study, the pharmacokinetic interaction of Ocimum sanctum hydroalcoholic leaf extract at 1000 mg/kg (OSHE) with levetiracetam (LEV), a new antiepileptic drug at 300 mg/kg (after 14 days of drug treatment) with pentylenetetrazole (PTZ) kindling (24 injections on alternate days and challenge for 7 days), was studied, and 85.72% protection was observed in the Ocimum-treated groups. Furthermore, the Ocimum-treated group exhibited better memory retention potential (as observed in the Morris water maze and passive avoidance test). The Ocimum-treated group also exhibited a lower oxidation stress potential. Histologically, the Ocimum-treated group exhibited the fewest histopathological changes. Furthermore, Ocimum treatment increased the Tmax. In another study employing Thai medicine, no growth inhibitory effect of Ocimum sanctum or Ocimum basilicum against Helicobacter pylori was found (MIC >512 μg/ml) [139].
Phyllanthus emblica
In one study, lyophilized aqueous extract of the fruits of Phyllanthus emblica at 100 mg/kg body weight per oral for 7 days significantly reduced indomethacin-induced ulceration (81% inhibition), enhanced catalase secretion, and reduced glutathione and malonaldehyde levels. The aqueous extract of Phyllanthus emblica (150-300 mg/kg dose) significantly ameliorated functional dyspepsia in a reserpine-induced model. It could increase the levels of gastrointestinal hormones such as motilin and gastrin as well as decrease the level of vasoactive intestinal peptide. Furthermore, studies have shown that this extract can regulate the microbiome structure and increase the abundance of good bacteria, consequently decreasing the abundance of bad bacteria [140]. Another study reported the efficacy of gallic acid extract of Phyllanthus emblica in indomethacin-induced ulcer healing by inducing PGE2 and elevating the NOS/iNOS (nitric oxide synthase/inducible nitric oxide synthase) ratio [141]. Another study reported the protective effect of the butanol extract of the water fraction of P. emblica fruits (100 kg/bw) on indomethacin-induced ulceration by enhancing mucin and hexosamine levels without altering malondialdehyde or superoxide dismutase levels [142]. In another study, the antiemetic effect of Embelica officinalis extract was investigated using a cisplatin-induced gastric emptying model, and b/w doses of 250-500 mg/kg significantly reversed cisplatin-induced gastric emptying and were found tobe promising agents against chemotherapy-induced nausea and vomiting. In a double-arm randomized, double-blind, placebo-controlled clinical trial of 68 patients with classic GERD symptoms (heartburn, regurgitation, epigastric pain), 500 mg of Amla tablets were administered twice daily after meals for 4 weeks, and the severity of regurgitation decreased significantly in the 68 group with a frequency of heartburn and regurgitation (NERD) [143]. Furthermore, at doses between 150 and 300 mg/kg, aq. phylanthus emblica (fruit) ameliorated functional dyspepsia in mice by increasing gastrointestinal motility, the gastric emptying rate and the small intestinal transit rate; elevating the levels of motilin and gastrin; and decreasing the level of vasoactive intestinal peptides (VIPs). The ethanolic extract of E. officinalis leaves exhibited an anti-H. pylori effect with an MIC of 10 μg/ml and produced an inhibition zone diameter of <3 cm [144]. In another study, gold nanoparticles composed of Phyllanthus emblica fruit extract (PEFE) and Phylanthus emblica fruit extract (PEFE) prepared from Bifidobacterium lactis exhibited cytotoxic activity in AGS cells by inducing apoptosis via the PTEN (Phosphatase and tensin homolog)-induced kinase 1 (PINKI)-perkin pathway-mediated reduction in mitochondrial membrane potential, inhibition of autophagy, downregulation of LC3-11/LC3-I (Microtubule-associated protein 1A/1B-light chain 3) and Beclin-1 expression, and upregulation of p62 expression [145]. Another study reported the isolation of norsesquiterpenoids, phenolic compounds and the main constituent proanthocyanidin (Figure 5, 16) polymers, all of which exhibited antiproliferative activity against B 16F10, HeLa, and MK-1 cell growth.
In another study, the methanolic extract of Emblica officinalis exhibited significant antiulcer activity in different gastric ulcer models in rats (aspirin, ethanol, cold-restrained stress, pylorus ligation, acetic acid-induced chronic ulceration) by reducing acid and pepsin secretion and enhancing mucus secretion, cellular mucus and the lifespan of mucous cells, as well as exhibiting antioxidant activity. The ulcer index decreased by 88.2%, the mucosal LPO (lipid peroxidation) decreased by 69%, the SOD decreased by 53.1%, and the mucosal CAT (catalase) increased by 59.8%. Another study reported the anti-GU activity of amla extract (250-500 mg/kg in rats) against pylorus ligation, indomethacin, hypothermic restraint stress-induced ulcers, and ethanol-induced ulcers. These compounds exhibited significant antiulcer activity, antisecretory activity, were cytoprotective against ethanol-induced depletion of stomach wall mucus, and reduced the nonprotein sulfhydryl concentration.
In another study, Kalpamrutha (KA), an indigenous Siddha formulation containing Semecarpus anacardium nett milk extract, the dried flower of Emblica officinalis fruit and honey exhibited significant antiulcer effects at a dose of 150 mg/kg. In another study, Triphala significantly reduced benzo-α-pyrene-induced forestomach papillomagenesis in rats: upon short-term treatment, a 77% reduction in tumor burden and 66-62% reduction were observed in a 2-5% Triphala-containing diet. Furthermore, the polyhedral formulation appeared more promising than the single component, indicating its putative chemopreventive potential. In one study with Nisha amlaki (NA: 200 mg/kg), a formulation with Curcuma longa Linn (Nisha in Sanskrit) and Phyllanthus emblica (Indian gooseberry) was assessed for pharmacokinetic and pharmacodynamic interactions with curcuminoids (CE:30 mg/kg) in normal and diabetic rats fed for 7 days. After 7 days, the feeding was continued with metformin (MET) for 15 days. The study of MET PK parameters, such as Cmax, AUC0-α, t1/2, CL, Vd, NA-MET and CA-MET, revealed that both Cmax, AUC0-α, and t1/2 increased while CL and Vd decreased. Furthermore, both combinations reduce plasma glucose, TG (triglyceride), and TC (t-cell count) levels. In diabetic rats, coadministration of NA-MET, as well as CE-MET, reduced the Cmax, AUC0-α, and t1/2 levels and enhanced the CL and Vd levels, indicating that the NA formulation exhibited potent antihypertensive and antidiabetic effects [146].
Aegle marmalos
In one study, the effect of an aqueous extract of ripe Aegle marmalos (AM) fruit pulp (250 mg/kg for 14 days) on gastric ulceration was evaluated. There was a significant reduction in the mean ulcer index, an increase in the enterochromaffin cell count and 5-HT (5-hydroxy tryptamine) content, and an increase in the adherent mucus content [147]. In another study, the gastroprotective effect of Aegle mermalos (200-800 mg/kg) on an ethanol-induced ulceration model was studied. Drug pretreatment reduced the ulcer index and provided 83% cytoprotection [148]. In another study, Aegle mermalos extract at concentrations as high as 100 mg/kg administered via the intraperitoneal route for 14 days did not exhibit any signs of toxicity, indicating a high margin of drug safety. In another study, Aegle marmelos unripe fruit extracts (50-100 mg/kg i.p.) prevented the castor oil-induced increase in intestinal transit as well as the accumulation of intestinal fluids and inhibited the contractile response in the guinea pig ileum invitro, indicating its potential as an antidiarrheal agent.
Another study reported the protective effect of Aegle mermalos active constituents (Marmelosin; Figure 6, 17)) on chromium-induced cytotoxicity in HepG2 cell lines, such as oxidation stress, apoptotic cell death, downregulation of markers such as Gadd45 (Growth arrest and DNA-damage-inducible protein), Bcl-2, Bax, and Caspase 3 gene expression, and inhibition of intracellular ROS (reactive oxygen species) and RNS (reactive nitrogen species). Another study reported the effect of 50-100 mg/kg Aegle mermalos on the levels of the carcinogen-metabolizing phase-I and phase-II enzymes, antioxidant enzymes, glutathione, lactate dehydrogenase, and lipid peroxidation for 14 days. The extract could efficiently induce glutathione S-transferase, DT-diaphorase, superoxide dismutase, and catalase in the lung; glutathione-S-transferase, DT-diaphorase and superoxide dismutase in the forestomach; and DT-diaphorase and superoxide dismutase in the lungs, indicating its chemopreventive potential against chemical carcinogenesis. Another study reported the significant antiulcer activity of a pyranocoumarin (Figure 6, 18) isolated from the seeds of Aegle marmelos against pylorus ligation and aspirin-induced gastric ulceration in rats and cold-restraint stress-induced gastric ulcers in rats and guinea pigs.The physiological efficacy of Aegeline (Figure 6, 19) (the major biologically active constituent of Aegle marmelos) was studied in mice following feeding at 30 mg/kg and 300 mg/kg, and a detailed analysis was performed using tissue homogenates of the liver, kidney, and brain. The results indicated that for gastrointestinal absorption in plasma had a Tmax value of 0.5 h (1.9 h in the liver), which was not detectable after 8 h, and t1/2 values of 1.4 h (1.2 in the liver) and 1.3h (1.7 h in the liver), respectively, at two doses [149].
Marketability of the plants
The demand for many of these medicinal plants in the market for medicinal plant products is high. These are used for manufacturing Ayurveda, Unani, Siddha and Homoeopathy medicines. A. marmelos, A. paniculata, A. indica, E. alba, E. officinalis, H. antidysenterica, T. cordifolia, T. belerica and T. chebula are used in formulations for gastrointestinal tract disorders. V. negundo, G. arborea, C. wightii and A. marmelos are common ingredients in medicines used to treat arthritis. A. scholaris, A. paniculata, and T. cordifolia are used as ingredients for antipyretic preparations. C. serratum, O. sanctum and E. officinalis are used in anti-asthmatic preparations. W. somnifera, T. cordifolia and E. officinalis are used as antioxidants and immunomodulators. R. serpentina, W. somnifera and T. arjuna are components of cardiovascular preparations. A. vera is a common ingredient in many cosmetic products.
During and after the COVID-19 pandemic, the demand for Ayurvedic immunomodulatory plant drugs such as W. somnifera, T. cordifolia, E. officinalis, O. sanctum and others increased many-fold. A recent survey reported the growth of Ayurvedic immune-boosting plant drugs after the pandemic. India is a major player in the market of medicinal plants. At present, the market size of medicinal plants in the global market is 165 billion USD, and it is expected to reach 345 billion USD by 2030 [152].
CONCLUSION
In this article, an intensive review of Indian medicinal plants is presented. Each plant was characterized by its morphology and medicinal and clinical importance. The entire article has documented the gastroprotective principle, anticancer potential, and anti-Helicobacter pylori effects of various herb (in some cases herb-drug interactions were presented). The pharmacokinetic profile also revealed the distinctions between different plants and envisioned the druggability of the active compounds present. From clinical perspective, among the six plants described in details, Phyllanthus embelica followed by Ocimum sanctum and Azadirachta Indica appeared much promising as gastroprotective. The active constituents explored are coumarins, alkaloids (steroidal, indole, isoquinoline, protoberberine), anthraquinones, terpenoids, limonoids, flavonoids, phytoestrogen, glycolipid, glycoprotein, saponins, lignan, sesquitepene lactones, ellagitannin, hydrolysable tannin, chlorogenic acid, depsides, coumestan, glycosides (terpenes, sarsapogenins), rhamnosides etc. In the post-Covid era, it is of utmost necessity to generate potential agents having dual activity against gastric disease as well as antimicrobial in general. In this review, a brief documentation on the gastroprotective potential of a number of plants are attempted, that may be useful for future exploration to generate new therapeutics that can benefit mankind, locally as well as globally.
REFERENCES
- Maconi G, Sainaghi M, Molteni M, Bosani M, Gallus S, Ricci G, et al. (2009). Predictors of long-term outcome of functional dyspepsia and duodenal ulcer after successful Helicobacter pylori eradication – a 7-year follow-up study. Eur J Gastroenterol Hepatol. 21:387-393.
- Goswami S. (2022). Interplay of potassium channel, gastric parietal cell and proton pump in gastrointestinal physiology, pathology and pharmacology. Minerva Gastroenterol (Torino). 68:289-305.
- Jonaitis P, Kupcinskas L, Kupcinskas J. (2021). Molecular Alterations in Gastric Intestinal Metaplasia. Int J Mol Sci. 22:5758 (1-12).
- Mascellino MT, Pontone S, Vega AE, Malfertheiner P. (2022). Editorial: Helicobacter pylori infection: pathogenesis, antibiotic resistance, advances and therapy, new treatment strategies. Front Microbiol. 13:1102144 (1-4).
- Smith SI, Ajayi A, Jolaiya T, Onyekwere C, Setshedi M, Schulz C, et al. (2022). Helicobacter pylori Infection in Africa: Update of the Current Situation and Challenges. Dig Dis. 40:535-544.
- Sjomina O, Pavlova J, Niv Y, Leja M. (2018). Epidemiology of Helicobacter pylori infection. Helicobacter. Suppl 1:e12514.
- Thomson ABR and Shaffer EA. (2012). First Principles of Gastroenterology and Hepatology. 2012.CAPstone (Canadian Academic Publishers Ltd).
- Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 33:1582-1614.
- Wang S, Dong G, Sheng C. (2019). Structural simplifications: an efficient strategy is lead optimization. Acta Pharm Sin B. 9: 880-901.
- Seide T, Wieder O, Garon A, Langer T. (2020). Application of pharmacophore concept in natural product inspired drug design. Mol Inf. 39:2000059.
- Sukmarini L. (2021). Recent advances in discovery of lead structures from microbial natural products: genomics -and metagenomics-guided acceleration. Molecules. 2542:1-13.
- Oselusi SO, Christoffels C, Egieyeh SA. (2021). Cheminformatic characterization of natural antimicrobial products for the development of new lead compounds. Molecules. 26: 3970.
- Maithani D, Sharma A, Gangola S, Chaudhary P, Bhatt P. (2022). Insight into applications and strategies for discovery of microbial bioactive metabolites. Microbiol Res. 261:127053.
- Holzmeyer L, Hartig AK, Franke K, Brandt W, Mueller-Riehl A, Wessjohann LA, et al. (2020). Evaluation of plant sources for anti-infective lead compound discovery by correlating phylogenetic, spatial, and bioactivity data. Proc Natl Acad Sci USA. 117:12444-12451.
- Zhu F, Qin C, Tao T, Liu X, Shi Z, Ma X, et al. (2011). Clustered patterns of species origins of nature-derived drugs and clues for future bioprospecting. Proc Natl Acad Sci USA. 108: 12943-12948.
- Junior CMS, Silva SMC, Sales EM, Velozo ES, Santos EKPD, Canuto GAB, et al. (2023). Coumarins from Rutaceae: Chemical diversity and biological activities. Fitoterapia. 168:105489.
- Bhadane BS, Patil MP, Maheshwari VL, Patil RH. (2018). Ethnopharmacology, phytochemistry, and biotechnological advances of family Apocynaceae: a review. Phytother Res. 32:1181-1210.
- Kumar P, Bhushan A, Gupta P, Gairola S. (2022). Comparative morpho-anatomical standardization and chemical profiling of root drugs for distinction of fourteen species of family Apocynaceae. Bot Stud. 63:12.
- Mohammed AE, Abdul-Hameed ZH, Alotaibi MO, Bawakid NO, Sobahi TR, Abdel-Lateff A, et al. (2021). Chemical diversity and bioactivities of monoterpene indole alkaloids (MIAs) from six Apocynaceae genera. Molecules. 26:488.
- Pecio Ł, Pecio S, Mroczek T, Oleszek W. (2023). Spiro-Flavonoids in Nature: A Critical Review of Structural Diversity and Bioactivity. Molecules. 28:5420.
- Bhat MN, Singh B, Surmal O, Singh B, Shivgotra V, Musarella CM. (2021). Ethnobotany of the Himalayas: Safeguarding medical practices and traditional uses of Kashmir regions. Biology (Basel). 10:851.
- Smýkal P, vonWettberg EJ, McPhee K. (2020). Legume genetics and biology: from Mendel’s pea to legume genomics. Int J Mol Sci. 21: 3336 (1-5).
- Ramalho SD, Pinto MEF, Ferreira D, Bolzani VS. (2018). Biologically active orbitides from the Euphorbiaceae family. Planta Med. 84:558-567.
- Amtaghri S, Akdad M, Slaoui M, Eddouks M. (2022). Traditional uses, pharmacological, and phytochemical studies of euphorbia: a review. Curr Top Med Chem. 22:1553-1570.
- Anokwuru CP, Sandasi M, Chen W, van Vuuren S, Elisha IL, Combrinck S, et al. (2021). Investigating antimicrobial compounds in South African Combretaceae species using a biochemometric approach. J Ethnopharmacol. 269: 113681.
- Yang SZ, Chen PH. (2016). Endocarps of menispermaceous plants in Taiwan. Bot Stud. 57:14.
- Wefferling KM, Hoot SB, Neves SS. (2013). Phylogeny and fruit evolution in Menispermaceae. Am J Bot. 100:883-905.
- Fiesel PD, Parks HM, Last RL, Barry CS. (2022). Fruity, sticky, stinky, spicy, bitter, addictive, and deadly: evolutionary signatures of metabolic complexity in the Solanaceae. Nat Prod Rep. 39:1438-1464.
- Jeiter J, Hilger HH, Smets EF, Weigend M. (2017). The relationship between nectaries and floral architecture: a case study in Geraniaceae and Hypseocharitaceae. Ann Bot. 120:791-803.
- Cassemiro NS, Sanches LB, Kato NN, Ruller R, Carollo CA, de Mello JCP, et al. (2021). New derivatives of the iridoid specioside from fungal biotransformation. Appl Microbiol Biotechnol. 105:7731-41.
- Gierlicka I, Kasprzyk I, Wnuk M. (2022). Imaging Flow Cytometry as a Quick and Effective Identification Technique of Pollen Grains from Betulaceae, Oleaceae, Urticaceae and Asteraceae. Cells. 11:598.
- Hines J, Pereira HM. (2021). Biodiversity:monitoring trends and implications for ecosystem functioning. Curr Biol. 31: R1390-1392.
- Ceausu S, Gomes I, Pereira HM. (2015). Conservation planning for biodiversity and wilderness: a real-world example. Environ Manage. 55:1168-1170.
- Brose U, Blanchard JL, Eklöf A, Galiana N, Hartvig M, Hirt MR, et al. (2017). Predicting the consequences of species loss using size-structured biodiversity approaches. Biol Rev Camb Philos Soc. 92:684-97.
- Vincent H, Hole D, Maxted N. (2022). Congruence between global crop wild relative hotspots and biodiversity hotspots. Biol Conserv. 265:109432.
- Bawa KS, Das A, Krishnaswamy J, Karanth KU, Kumar NS, Rao M. (2007). Western Ghats & Sri Lanka Biodiversity Hotspot. 2007:1-100.
- Leknettip S, Chawchai S, Choowong M, Mueller D, Fűlling A, Preusser F. (2023). Sand ridges from the coastal zone of southern Thailand reflect quaternary sea-level history and environmental condition in Sundaland. Quat Sci Rev. 316: 108264.
- Mantiquilla JA, Shiao MS, Shih HC, Chen WH, Chiang YC. (2021). A review on the genetic structure of ecologically and economically important mangrove species in the Indo-West pacific. Ecol Genet Genom. 18:100078.
- Raes N, Saw LG, van Welzen PC, Yahara T. (2013). Legume diversity as indicator for botanical diversity on Sundaland, South East Asia. S Afr J Bot. 89:265-272.
- Gooriah LD, Davidar P, Chase JM. (2020). Species-area relationships in the Andaman and Nicobar islands emerge because rarer species are disproportionately favoured on larger islands. Ecol Evol. 10:7551-7559.
- Chander MP, Kartick C, Vijayachari P. (2015). Ethnomedicinal knowledge among karens of Andaman &Nicobar islands, India. J Ethnopharmacol. 162:127-133.
- Gosai KR, Parmar R. (2017). A clinical trial to evaluate efficacy of Saptaparna (Alstonia Scholaris Linn.) Twak Churna in Vicharchika (Eczema). JAIMS. 2:54-57.
- Khyade MS , Kasote DM , Vaikos NP. (2014). Alstonia scholaris (L.) R. Br. and Alstonia macrophylla Wall. ex G. Don: A comparative review on traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 153:1-18.
- Mitra SK, Prakash NS, Sundaram R. (2012). Shatavarins (containing Shatavarin IV) with anticancer activity from the roots of Asparagus racemosus. Indian J Pharmacol. 44:732-736.
- Bhandari P, Sendri N, Devidas SB. (2020). Dammarane triterpenoid glycosides in Bacopa monnieri: a review on chemical diversity and bioactivity. Phytochemistry. 172:112276
- Murthy HN. (2022). Biotechnological production of bacosides from cell and organ cultures of Bacopa monnieri. Appl Microbiol Biotechnol. 106:1799-1811.
- Sharma N, Sharma A, Bhatia G, Landi M, Brestic M, Singh B, et al. (2019). Isolation of Phytochemicals from Bauhinia variegata L. Bark and Their In Vitro Antioxidant and Cytotoxic Potential. Antioxidants (Basel). 8:492.
- Mishra T, Arya RK, Meena S, Joshi P, Pal M, Meena B, et al. (2016). Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis bark. Plos One. 11:e0159430.
- Kumar S, Singh B, Singh R. (2022). Catharanthus roseus (L.) G. Don: A review of its ethnobotany, phytochemistry, ethnopharmacology and toxicities. J Ethnopharmacol. 284:114647.
- Khare CP (ed). Indian Medicinal Plants. An Illustrated Dictionary. Springer-Verlag Berlin/Heidelberg. 159.
- Sung NY, Kim MY, Cho JY. (2015). Scutellarein Reduces Inflammatory Responses by Inhibiting Src Kinase Activity. Korean J Physiol Pharmacol. 19: 441-449.
- Batiha GE, Wasef L, Teibo JO, Shaheen HM, Zakariya AM, Akinfe OA, et al. (2023). Commiphora myrrh: a phytochemical and pharmacological update. Naunyn Schmiedebergs Arch Pharmacol. 396:405-420.
- Priya SKS, Jha A, Kumar RCS, Sabarathinam S. (2022). The role of guggulsterone on the NF-κB pathway in inflammatory bowel disease: preclinical evidence. Future Sci OA. 8:FSO803.
- Rosenthal R, Luettig J, Hering NA, Krug SM, Albrecht U, Fromm M, et al. (2017). Myrrh exerts barrier-stabilizing and -protective effects in HT-29/B6 and Caco-2 intestinal epithelial cells. Int J Colorectal Dis. 32:623-634.
- Vissiennon C, Goos KH, Arnhold J, Nieber K. (2017). Mechanisms on spasmolytic and anti-inflammatory effects of a herbal medicinal product consisting of myrrh, chamomile flower, and coffee charcoal. Wien Med Wochenschr. 167:169-176.
- Weber L, Kuck K, Jürgenliemk G, Heilmann J, Lipowicz B, Vissiennon C. (2020). Anti-Inflammatory and Barrier-Stabilizing Effects of Myrrh, Coffee Charcoal and Chamomile Flower Extract in a Co-Culture Cell Model of the Intestinal Mucosa. Biomolecules. 10:1033.
- Dangol S, Poudel DK, Ojha PK, Maharjan S, Poudel A, Satyal R, et al. (2023). Essential Oil Composition Analysis of Cymbopogon Species from Eastern Nepal by GC‒MS and Chiral GC‒MS, and Antimicrobial Activity of Some Major Compounds. Molecules. 28:543.
- Timalsina D, Devkota HP. (2021). Eclipta prostrata (L.) L. (Asteraceae): Ethnomedicinal Uses, Chemical Constituents, and Biological Activities. Biomolecules. 11:1738.
- Deng YT, Kang WB, Zhao JN, Liu G, Zhao MG. (2015). Osteoprotective Effect of Echinocystic Acid, a Triterpone Component from Eclipta prostrata, in Ovariectomy-Induced Osteoporotic Rats. PLoS One. 10:e0136572.
- Kim DS, Kim SH, Kee JY, Han YH, Park JB, Mun JG, et al. (2017). Eclipta prostrata Improves DSS-Induced Colitis through Regulation of Inflammatory Response in Intestinal Epithelial Cells. Am J Chin Med. 45:1047-1060.
- Lawrence L, Menon S, Vincent S, Sivaram VP, Padikkala J. (2016). Radical scavenging and gastroprotective activity of methanolic extract of Gmelina arborea stem bark. J Ayurveda Integr Med. 7:78-82.
- Upadhya V, Hegde HV, Bhat S, Hurkadale PJ, Kholkute SD, Hegde GR. (2012). Ethnomedicinal plants used to treat bone fracture from North-Central Western Ghats of India. J Ethnopharmacol. 142: 557-662.
- Johari S, Gandhi T. (2016). A Randomized Single Blind Parallel Group Study Comparing Monoherbal Formulation Containing Holarrhena Antidysenterica Extract with Mesalamine in Chronic Ulcerative Colitis Patients. Anc Sci Life. 36:19-27.
- Sinha S, Sharma A, Reddy H, Rathi B, Prasad NVSRK, Vashishtha A. (2013). Evaluation of phytochemical and pharmacological aspects of Holarrhena antidysenterica (Wall.): A comprehensive review. J Pharm Res. 6:488-492.
- Ellena R, Quave CL, Pieroni A. (2012). Comparative medical ethnobotany of the senegalese community living in turin (northwestern Italy) and in adeane (southern senegal). Evid Based Complement Alternat Med. 2012:604363.
- Semwal RB, Semwal DK, Combrinck S, Cartwright-Jones C, Viljoen A. (2014). Lawsonia inermis L. (henna): ethnobotanical, phytochemical and pharmacological aspects. J Ethnopharmacol. 155:80-103.
- Mohammed DM, Ahmed KA, Desoukey MA, Sabry BA. (2022). Assessment of the antiulcer properties of Lawsonia inermis L. leaves and its nanoformulation against prolonged effect of acute ulcer in rats. Toxicol Rep. 9:337-345.
- Shitiz K, Gupta SP. (2021). Himalayan Medicinal Plants. Advances in Botany, Production & Research. 111-149.
- Sulaiman CT, Jyothi CK, Unnithan JKG, Prabhukumar KM, Balachandran I. (2020). Identification of validated substitute for Asoka (Saraca asoca (Roxb.) Willd.) by phytochemical and pharmacological evaluations.
- Devi RS, Kist M, Vani G, Devi CSS. (2008). Effect of methanolic extract of Terminalia arjuna against Helicobacter pylori 26695 lipopolysaccharide-induced gastric ulcer in rats. J Pharm Pharmacol. 60:505-514.
- Silva O, Viegas S, Mello-Sampayo CD, Costa MJP, Serrano R, Cabrita J, et al. (2012). Anti-Helicobacter pylori activity of Terminalia macroptera root. Fitoterapia. 83:872-876.
- Muhammad S, Khan BA, Akhtar N, Mahmood T, Rasul A, Hussain I, et al. (2012). The morphology, extractions, chemical constituents and uses of Terminalia chebula: A review. J Med Plant Res. 6:4772-4775.
- Patel AG, Patel MR, Nariya MB. (2018). Assessment of Trisama, an ayurvedic formulation on intestinal transit time in swiss albino mice. Ayu. 39:46-49.
- Wannasiri S, Jaijoy K, Chiranthanut N, Soonthornchareonnon N, Sireeratawong S. (2015). Effect of Tri-sa-maw recipe on gastrointestinal regulation and motility. J Med Assoc Thai. 98:Suppl 2, S1.
- Kaur M, Singh A, Kumar B. (2014). Comparative antidiarrheal and antiulcer effect of the aqueous and ethanolic stem bark extracts of Tinospora cordifolia in rats. J Adv Pharm Technol Res. 5:122-128.
- Sharma P, Bharat P, Dwivedee BP, Bisht D, Dash AK, Kumar D. (2019). The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon. 5:e02437.
- Zaware BB, Nirmal SA, Baheti DG, Patil AN, Mandal SC. (2011). Potential of Vitex negundo roots in the treatment of ulcerative colitis in mice. Pharm Biol. 49:874-878.
- Koirala N, Dhakal C, Munankarmi NN, Ali SW, Hameed A, Martins N, et al. (2020). Vitex negundo Linn.: phytochemical composition, nutritional analysis, and antioxidant and antimicrobial activity. Cell Mol Biol (Noisy-le-grand). 66:1-7.
- Kaushik D, Yadav J, Kaushik P, Sacher D, Rani R. (2011). Current pharmacological and phytochemical studies of the plant Alpinia galanga. Zhong Xi Yi Jie He Xue Bao. 9:1061-1065.
- Wang SY, Cai L, Yang N, Xu FF, Wu YS, Liu B. (2023). Chemical composition of the Kaempferia galanga L. essential oil and its in vitro and in vivo antioxidant activities. Front Nutr. 10:1080487.
- Taslima NA, Djide MN, Rifai Y, Syahruddin AN, Rampo YR, Mustamin M, et al. (2019). Double-blind randomized clinical trial of Kaempferia galangal L extract as an anti-inflammation (prostaglandin E2 and tumor necrosis factor alpha) on osteoarthritis. Asian J Pharm Clinic Res. 12:63-66.
- Nam HH, Choo BK. (2021). Geranium koreanum, a medicinal plant Geranii Herba, ameliorate the gastric mucosal injury in gastritis-induced mice. J Ethnopharmacol. 265:113041.
- Lee H, Kim W, Kang HG, Kim WJ, Lee SC, Kim SJ. (2020). Geranium thunbergii extract-induced G1 phase cell cycle arrest and apoptosis in gastric cancer cells. Anim Cells Syst (Seoul). 24:26-33.
- Bawish BM, Rabab MA, Gohari ST, Khattab MS, AbdElkader NA, Elsharkawy SH, et al. (2023). Promising effect of Geranium robertianum L. leaves and Aloe vera gel powder on Aspirin®-induced gastric ulcers in Wistar rats: anxiolytic behavioural effect, antioxidant activity, and protective pathways. Inflammopharmacology. 31:3183-3201.
- Verma RS, Padalia RC, Chauhan A. (2013). Fragrant volatile oil composition of Nutmeg Geranium (Pelargonium × fragrans Willd.) from India. Nat Prod Res. 27: 761-766.
- Zhang XQ, Gu HM, Li XZ, Xu ZN, Chen YS, Li Y. (2013). Anti-Helicobacter pylori compounds from the ethanol extracts of Geranium wilfordii. J Ethnopharmacol. 147:204-207.
- Hamann FR, Brusco I, Severo GdC , Carvalho LM, Faccin H, Gobo L. (2019). Mansoa alliacea extract presents antinociceptive effect in a chronic inflammatory pain model in mice through opioid mechanisms. Neurochem Int. 122:157-169.
- Kashyap D, Roy R, Varshney N, Baral B, Bagde PH, Kandpal M, et al. (2023). Withania somnifera extract reduces gastric cancerous properties through inhibition of gankyrin in cellular milieu produced by Helicobacter pylori and Epstein Barr virus. J Biomol Struct Dyn. 1-17.
- Liu Y, Wang C, Wu J, Tan L, Gao P, Wu S, et al. (2023). Study on the Comprehensive Phytochemicals and the Anti-Ulcerative Colitis Effect of Saussurea pulchella. Molecules. 28:1526.
- Bandyopadhyay U, Biswas K, Sen Gupta A, Moitra P, Dutta P, Sarkar D et al. (2004). Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci. 75:2867-2878.
- Blum FC, Singh J, Merrell DS. (2019). In vitro activity of neem (Azadirachta Indica) oil extract against Helicobacter pylori. J Ethnopharmacol. 232:236.
- Alzohairy MA. (2016). Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid Based Complement Alternat Med. 2016:7382506.
- Saleem S, Muhammad G, Hussain MA, Bukhari SNA. (2018). A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother Res. 32:1241-1272.
- Sarkar S, Singh RP, Bhattacharya G. (2021). Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: an update on molecular approach. 3 Biotech. 11:178.
- Gangar SC, Sandhir R, Koul A. (2010). Effects of Azadirachta Indica on certain hematogical parameter during benzo(a)pyrene induced murine forestomach tumorigenesis. Eur Rev Med Pharmacol Sci. 14:1055-1072.
- Ofusori DA, Falana BA, Ofusori AE, Caxton-Martins EA. (2010). Regenerative potential of aqueous extract of neem Azadirachta Indica on the stomach and ileum following ethanol- induced mucosa lesion in adult wistar rats. Gastroenterology Res. 3:86-90.
- He Z, Jiang C, Zhang J, Yin Z, Yin ZF, Zhu YF, et al. (2016). Neem tree (Azadirachta Indica) extract specifically suppress the growth of tumors in H22- bearing Kunming mice. Z Naturforsch CJ Biosci. 71:201-208.
- Paul R, Prasad M, Sah NK. (2011). Anticancer biology of Azadirachta indica L (neem): a mini review. Cancer Biol Ther. 12:467-476.
- Jain CK, Pradhan BS, Banerjee S, Mondal NB, Majumder SS, Bhattacharyya M, et al. (2015). Sulfonoquinovosyl diacylglyceride selectively targets acute lymphoblastic leukemia cells and exerts potent anti-leukemic effects in vivo. Sci Rep. 5:12082.
- Singh R, Mishra V, Pandeti S, Palit G, Barthwal MK, Pandey HP, et al. (2015). Cytoprotective and anti-secretory effects of azadiradione isolated from the seeds of Azadirachta Indica (neem) on gastric ulcers in rats model. Phytother Res. 29:910-916.
- Manosroi A, Kitdamrongtham W, Ishii K, Shinozaki T, Tachi Y, Takagi M, et al. (2014). Limonoides from Azadirachta Indica vas. siamensis extracts and there cytotoxic and melanogenesis-inhibitory activities. Chem Biodivers. 11:505-531.
- Yadav DK, Bharitkar VP, Hazra A, Pal U, Verma S, Jana S, et al. (2017). Tamarixetin 3-0-β-d- glucopyranoside from (Azadirachta Indica) leaves: gastroprotective role through inhibition of matrix metalloproteinase-9 activity in the mice. J Nat Prod. 80:1347-1353.
- Zhou H, Li F, Li Y. (2022). Anticancer activity of gedunin by induction of apoptosis in human gastric cancer AGS cells. Appl Biochem Biotechnol. 194: 5322-5332.
- Bharitkar YP, Bathini S, Ojha D, Ghosh S, Mukherjee H, Kuotsu K, et al. (2014). Antibacterial and antiviral evaluation of sulfonoquinovosyldiacylglyceride: a glycolipid isolated from Azadirachta indica leaves. Lett Appl Microbiol. 58:184-189.
- Chatterjee R, Singh O, Pachuau L, Malik SP, Paul M, Bhadra K, et al. (2010). Identification of a sulfonoquinovosyldiacylglyceride from Azadirachta indica and studies on its cytotoxic activity and DNA binding properties. Bioorg Med Chem Lett. 20:6699-6702.
- Fernandes SR, Barreiros L, Oliveira RF, Cruz A, Prudêncio C, Oliveira AI, et al. (2019). Chemistry, bioactivities, extraction and analysis of azadirachtin: State-of-the-art. Fitoterapia. 134:141-150.
- Kurimoto SI, Takaishi Y, Ahmed FA, Kashiwada Y. (2014). Triterpenoids from the fruits of Azadirachta indica (Meliaceae). Fitoterapia. 92:200-205.
- Kushwaha P, Khedgikar V, Haldar S, Gautam J, Mulani FA, Hirekodathakallu V, et al. (2016). Azadirachta indica triterpenoids promote osteoblast differentiation and mineralization in vitro and in vivo. Bioorg Med Chem Lett. 26:3719-3724.
- Tahir A, Kampleitner C, Wirglauer T, Grienke U, Hoffmann O, Rollinger JM. (2022). Biochemometry-based discovery of phenylpropanoids from Azadirachta indica fruits as inhibitors of in vitro osteoclast formation. Molecules. 27:3611.
- Adepiti AO, Adeagbo BA, Adehin A, Bolaji, OO, Elujoba AA. (2020). Influence of MAMA decoction, an herbal antimalarial, on the pharmacokinetics of amodiaquine in mice. Eur J Drug Metab Pharmacokinet. 45:81-88.
- Baira SM, Khurana A, Somagoni J, Srinivas R, Godugu C, Talluri MVNK. (2018). First report on the pharmacokinetic profile of nimbolide, a novel anticancer agent in oral and intravenous administered rat by LC/MS method. J Chromatogram B Analyt Technol Biomed Life Sci. 1092:191-198.
- Hussein J, El-Bana MA, El-Naggar ME, Abdel-Latif Y, El-Sayed SM, Medhat D. (2021). Prophylactic effect of probiotics fortified with Aloe Vera pulp nanoemulsion against ethanol-induced gastric ulcer. Toxicol Mech Methods. 31:699-710.
- Yahya R, Al- Rajhi AMH, Alzaid S Z, Al-Abboud MA, Almuhayawi MS, Al-Jaouni SK, et al. (2022). Molecular docking and efficacy of aloe vera gel based on chitosan nanoparticles against Helicobacter pylori and its antioxidant and anti-inflammatory activity. Polymers (Basel). 14:2994.
- Cellini L, Dibartolomeo S, Di-campli E, Genovese S, Locatelli M, Di giulio M. (2014). In vitro activity of aloe vera inner gel against Helicobacter pylori strains. Lett Appl Microbiol. 59:43-48.
- Ranade AN, Ranpise NS, Ramesh C. (2014). Exploring the potential of gastro retentive dosage form in delivery of ellagic acid and aloe vera gel powder for treatment of gastric ulcers. Curr Drug Deliv. 11:287-297.
- Ranade AN, Wankhede SS, Ranprise NS, Mundada MS. (2012). Development of bilayer floating tablet amoxicillin and aloe vera gel powder for treatment of gastric ulcer. AAPS Pharm SciTech. 13:1518-1523.
- Al-Gabri N, Elnagar GM, Saghir SAM, El-Shaibany A, Alnomasy SF, Althafar ZM, et al. (2022). Preliminary study of gastroprotective effect of aloe perryi and Date Palm Extracts on pyloric ligation- induced gastric ulcer in experiment rats. Biomed Res Int. 2022:9246785.
- Boudreau MD, Beland FA, Nichols JA, Pogribna M. (2013). Toxicology and carcinogenesis studies of a nondecolorized (corrected) whole leaf extract of Aloebarbadensis miller (Aloe Vera) in F344/N rats and B6C3F1 mice (drinking water study). Natl Toxicol Program Tech Rep Ser. 577:1-266.
- Tang R, Zhang J, Nan H, Lv R, Chen XH, Liu Y, et al. (2022). Exploring molecular mechanism of Aloe barbadensis miller on Diphenoxylate-induced constipation in mice. Evid Based Complement Alternat Med. 2022; 6225758.
- Bahrami G, Malekshahi H, Miraghaee S, Madani H, Babaei A, Mohammadi B, et al. (2020). Protective and Therapeutic Effects of Aloe Vera Gel on Ulcerative Colitis Induced by Acetic Acid in Rats. Clin Nutr Res. 9:223-234.
- Asadi-Shahmirzadi A, Mozaffari S, Sanei Y, Baeeri M, Hajiaghaee R, Monsef-Esfahani HR, et al. (2012). Benefit of Aloe Vera and Matricaria recutita mixture in rat irritable bowel syndrome: Combination of antioxidant and spasmolytic effects. Chin J Integr Med.
- Chihara T, Shimpo K, Beppu H, Yamamoto N, Kaneko T, Wakamatsu K, et al. (2015). Effects of Aloe- emodin and Emodin on proliferation of the MKN45 human gastric- cancer cell line. Asian Pac J Cancer Prev. 16:3887-3891.
- Tao H, Tang T, Wang S, Wang Z, Yunfei Ma, Cai T, et al. (2019). The molecular mechanism of Aloin induce gastric cancer cells apoptosis by targeting high mobility group box 1. Drug Des Devel Ther. 13:1221-1231.
- Niu C, Ye WJ, Cui X, Sun J, Xiao S, Chen G, et al. (2020). UHPLC-MS/MS method for the quantification of aloin A in rat plasma and its application to a pharmacokinetic study. J Pharm Biomed Anal. 178:112928.
- Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. (2020). Pharmacological update properties of Aloe vera and its major active constituents. Molecules. 25:1324.
- Wasman SQ, Mahmood AA, Chua LS, Alshawsh MA, Hamdan S. (2011). Antioxidant and gastroprotective activities of Andrographis Paniculata (HempeduBumi) in Sprague Dawley rats. Indian J. Exp Biol. 49:767-772.
- Panneerselvam S, Arumugam G. (2011). A biochemical study on the gastroprotective effect of hydroalcoholic extract of Andrographis paniculata in rats. Indian J Pharmacol. 43:402-408.
- Das B, Chowdhury C, Kumar D, Sen R, Roy R, Das P, et al. (2010). Synthesis, cytotoxicity, and structure-activity relationship (SAR) studies of andrographolide analogues as anticancer agent. Bioorg Med Chem Lett. 20:6947-6950.
- Preet R, Chakraborty B, Siddharth S, Mohapatra P, Das D, Satapathy SR, et al. (2014). Synthesis and biological evaluation of andrographolide analogues as anticancer agents. Eur J Med Chem. 85:95-106.
- Pfisterer PH, Rollinger JM, Schyschka L, Rudy A, Vollmar AM, Stuppner H. (2010). Neoandrographolide from Andrographis paniculata as a potential natural chemosensitizer. Planta Med. 76:1698-1700.
- Malat P, Ekalaksananan T, Heawchaiyaphum C, Suebsasana S, Roytrakul S, Yingchutrakul Y, et al. (2021). Andrographolide inhibits lytic reactivation of Epstein‒Barr virus by modulating transcription factors in gastric cancer. Microrganisms. 9:2561.
- Ye L, Wang T, Tang L, Liu W, Yang Z, Zhou J, et al. (2011). Poor oral bioavailability of a promising anticancer agent andrographolide is due to extensive metabolism and efflux by P-glycoprotein. J Pharm Sci. 100:5007-5017.
- Saranya P, Geetha A, Selvamathy SMKN. (2011). A biochemical study on the gastroprotective effect of andrographolide in rats induced with gastric ulcer. Indian J Pharm Sci. 73:550-507.
- Lim SC, Jeon HJ, Kee KH, Lee MJ, Hong R, Han SL. (2017). Andrographolide induces apoptotic and nonapoptotic death and enhances tumor necrosis factor related apoptosis-inducing ligand-mediated apoptosis in gastric cancer cells. Oncol Lett. 13:3837-3844.
- Mandal S, Das DN, De K, Ray K, Roy G, Chaudhuri SB, et al. (1993). Ocimum sanctum linn–a study on gastric ulceration and gastric secretion in rats. Indian J Physiol Pharmacol. 37:91-92.
- Boonyanugomol W, Rukseree K, Prapatpong P, Reamtong O, Baik SC, Jung M, et al. (2021). An in vitro anticancer activity of Ocimum tenuiflorum essential oil by inducing apoptosis in human gastric cancer cell line. Medicina (Kaunas). 57:784.
- Mondal S, Varma S, Bamola VD, Naik SN, Mirdha BR, Padhi MM, et al. (2011). Double- blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum linn) leaf extract on healthy volunteers. J. Ethnophramacol. 136:452-456.
- Eleje GU, Ogbunugafor HA, Emegoakor CD, Okoye EI, Ezejiofor OI, Chukwurah SN, et al. (2019). Efficacy and safety of syferol-IHP for the treatment of peptic ulcer disease: a pilot, double-blind randomized trial. Clin Exp Gastroenterol. 12:21-30.
- Chaichanawongsaroj N, Amonyingcharoen S, Pattiyathanee P, Vilaichone RK, Poovorawan Y. (2012). Anti-Helicobacter pylori and anti-internalization activities of Thai folk remedies used to treat gastric ailments. J Med Plant Res. 6:1389-1393.
- Li XQ, Lin Y, Jiang Y, Wu BB, Yu Y. (2022). Aqueous extract of Phyllanthus emblica L. alleviates functional dyspepsia through regulating gastrointestinal hormones and gut microbiome in vivo. Foods. 11:1491.
- Chatterjee A, Chatterjee S, Biswas A, Bhattacharya S, Chattopadhyay S, Bandyopadhyay SK. (2012). Gallic acid enriched fraction of Phyllanthus emblica potentiates indomethacin-induced gastric ulcer healing via e-NOS-dependent pathway. Evid based Complement Alternate Med. 487380.
- Ahmad B, Hafeez N, Rauf A, Bashir S, Linfang H, Rehman MU, et al. (2021). Phyllanthus emblica: A comprehensive review of its therapeutic benefits. S AfrJ Bot. 138:278-310.
- Varnosfaderani SK, Hashem-Dabaghian F, Amin G, Bozorgi M, Heydarirad G, Nazem E, et al. (2018). Efficacy and safety of amla (Phyllanthus emblica L.) in nonerosive reflux disease: a double-blind, randomized, placebo-controlled clinical trial. J Integr Med. 16:126-131.
- Franklin A, Rao UA, Vijayakumar R, Srikumar R. (2012). In vitro Anti-Helicobacter Pylori Activity of Emblica officinalis. Int J Microbiol Res. 3:216-220.
- Wang R, Xu XY, Puja AM, Perumalsamy H, Balusamy SRD, Kim H, et al. (2021). Gold nanoparticles prepared with Phyllanthus emblica fruit extract and bifidobacterium animalis subsp. lactis can induce apoptosis via mitochondrial impairment with inhibition of autophagy in the human gastric carcinoma cell line AGS. Nanomaterials (Basel). 11:1260.
- Shengule S, Kumbhare K, Patil D, Mishra S, Apte K, Patwardhan B. (2018). Herb-drug interaction of Nisha Amalaki and Curcuminoids with metformin in normal and diabetic condition: A disease system approach. Biomed Pharmacother. 101:591-598.
- Singh P, Dutta SR, Guha D. (2015). Gastric mucosal protection by aegle marmelos against gastric mucosal damage: role of enterochromaffin cell and serotonin. Saudi J Gastroenterol. 21:35-42.
- Imran H, Sohail T, Rehman AU, Syed S. (2022). Dry ripe fruit of Aegle marmelos as anti-ulcer agent against ethanol induced gastric mucosal injury. Pak J. Pharm Sci. 35:1677-1682.
- Manda VK, Haron MH, Nir TM, Avula M, Ashfaq MK, Haider S. (2019). Pharmacokinetics and tissue distribution of Aegeline after oral administration in mice. Planta Med. 85:491-495.
- Sharma N, Radha, Kumar M, Zhang BH, Kumari N, Singh D, et al. (2022). Aegle marmelos (L) correa: an underutilized fruit with high nutraceutical values: a review. Int J Mol Sci. 23:10889.
- Chakthong S, Weaaryee P, Puangphet P, Mahabusarakam W, Plodpai P, Voravuthikunchai SP, et al. (2012). Alkaloid and coumarins from the green fruits of Aegle marmelos. Phytochemistry. 75:108-113.
- Global Medicinal Herbs Market – Industry Trends and Forecast to 2030. Available in https://www.databridgemarketresearch.com/reports/global-medicinal-herbs-market. Searched on 31/12/2023.