Previous Issues Volume 8, Issue 1 - 2024
Evaluation of the Toxic Potentials and Histopathological Variations in Clarias gariepinus Fingerlings Exposed to Ethanolic Extract of Costus afer
Essien-Ibok MA1, George UU2,*, Abiaobo NO3, Mbong EO4
1Department of Fisheries and Aquatic Environmental Management, University of Uyo, Nigeria
2Department of Fisheries and Aquaculture, Faculty of Agriculture, Akwa Ibom State University, Obio Akpa Campus, Akwa Ibom State, Nigeria
3Department of Zoology, Akwa Ibom State University, Ikot Akpaden, Nigeria
4Department of Environmental Biology, School of Science and Technology, Heritage Polytechnic-Eket, Akwa Ibom State, Nigeria
*Corresponding Author: George UU, Department of Fisheries and Aquaculture, Faculty of Agriculture, Akwa Ibom State University, Obio Akpa Campus, Akwa Ibom State, Nigeria; Email: [email protected]
Received Date: April 15, 2024
Publication Date: May 07, 2024
Citation: Essien-Ibok MA, et al. (2024). Evaluation of the Toxic Potentials and Histopathological Variations in Clarias gariepinus Fingerlings Exposed to Ethanolic Extract of Costus afer. Mathews J Cytol Histol. 8(1):28.
Copyright: Essien-Ibok MA, et al. © (2024)
ABSTRACT
This study investigates the toxicity of Costus afer on the histopathology of the gills of Clarias gariepinus fingerlings. Results indicates a concentration-dependent pattern of mortality, with significant reduction observed over time of exposure. Experimental groups exposed to C. afer exhibited pathological changes in the gills and behavioural abnormalities. Additionally, variations in water quality were observed following the introduction of the extract. The calculated LC50 of C. afer was determine to be 20.5 mg/l, providing crucial insights into the potential ecological impact of this plant extract on aquatic organisms.
Keywords: Costus afer, Clarias gariepinus, Toxicity, Histopathological changes, Fingerlings
INTRODUCTION
Fish and fish product demand is very high compared to the quantity supplied all over the world (Dada, et.al., 2019) [1]. Fish is a source of protein that is moderately cheap, available, easily processed and serves as a source of income compared to other livestock (Otoh et al., 2024 a, b) [2,3]. Globally, the demand for fish is at daily increased as the world population continue to expand whereas the supply of fish and fish products from the wild is drastically reduced due to environmental challenges and degradations leaving behind aquaculture as the only alternative source to achieving maximum production of fish to meet the protein intake of the growing human population (Otoh et. al 2024 c, d) [4,5].
Fish are ideal sentinels for detecting and monitoring aquatic pollutants or contaminants in aquatic environments (Bala, et. al., 2015) [6]. African cat fish (Clarias gariepinus) belongs to family Clariidae. It remained the most culturable species of significant in Nigeria and beyond (Otoh and Udoh, 2018) [7]. This is due to the unique characteristic of the species such as fast growth rate, good taste, generally accepted for consumption, high stocking density, high market price and high resistance to disease and ability to reproduce in captivity. (Nlewadim, et. al., 2011; Nya, et. al., 2017; 2023 a, b; Otoh, et. al., 2022) [8-12]. It is commonly and extensively cultured in man-made ponds and also occurs naturally in Nigerian natural freshwater systems.
Pollution leads to decrease in wild fisheries supplied (Adedeji and Okocha, 2011) [13]. Plant derivatives used for catching fishes are more friendly to the aquatic ecosystem and are proved economical compared to synthetic pesticides due to its ability to biodegrade (Das et. al., 2018) [14]. However, traditional fishing with plant substances and its derivatives has been shown to have negative consequences on aquatic biota, which roots to a significant ecological balance and does not encourage continuous fishing (Das et. al., 2018) [14]. Different plant species have been used arbitrarily to catch fish in lentic and lotic water bodies (Das et. al., 2018) [14]. Roots, barks, leaves, fruits and seeds are toxic parts of plants employed as fish poisons by artisanal fisherfolks (Ekpo, et. al., 2017) [15].
Water quality parameters are an integral part for the sustenance and survival of fish. Aquatic organisms are stressed due to alterations in water quality parameters (Michael et al., 2015; Ekpo et al., 2015; George and Atakpa, 2015 a, b) [16-19]. Standard water quality parameters are crucial for aquatic organisms’ survival and continuity (Jonah et al., 2020; George et. al., 2020 a, b) [20-22]. Deterioration in the water quality due to the presence of impurities or contaminants may ultimately reduce the growth rate, reproductive behaviour, resistance to disease making the animals vulnerable to diseases, thereby resulting in massive mortality of aquatic animals (George and Effiom, 2018, Jonah and George, 2019; Jonah et al., 2019) [23-25].
Costus afer commonly known as African ginger or bush cane is a perennial herbaceous plant native to tropical regions of West Africa. It belongs to the family Costaceae and is characterized by its distinctive red cone-shaped inflorescence and lance-shaped leaves. C. afer typically, grows up to 2 meters in height and has thick erect stems (Okwu and Okwu, 2004; Anaga et al., 2004) [26,27].
Costus afer extracts may contain compounds that are toxic to aquatic organisms such as fish, invertebrates and algae. Exposure to these compounds can lead to reduced survival, growth and reproduction rate, as well as physiological and behavioral changes. High concentrations of Costus afer extract may disrupt the balance of aquatic communities by selectively impacting certain species over others. This could lead to shift in species composition and diversity, potentially affecting ecosystem functioning.
Decomposition of Costus afer extract in water bodies could alter water quality parameters such as pH, dissolved oxygen and nutrients concentrations. This may have cascading effects on aquatic organisms and overall ecosystem health. In areas where Costus afer grows abundantly and is introduced to the aquatic ecosystem, it may alter habitat structure by shading out native vegetation or changing sediment composition. This can impact the availability of food and shelter for aquatic organisms, ultimately affecting their abundance and distribution.
The impacts of C. afer on aquatic ecosystems highlights the importance of understanding its potential toxicity and implementing measures to mitigate its introduction and spread into natural water bodies. There is paucity of literature on the acute toxicity effects of ethanolic extracts of C. afer on African catfish (C. gariepinus) fingerlings. Understanding the effects of Costus afer on C. gariepinus fingerlings will help ensure compliance with regulatory standards and guidelines. Studying the toxicological effects of Costus afer on C. gariepinus will contributes to scientific understanding of how different substances interact with aquatic organisms. This knowledge can inform future research and conservation efforts.
MATERIAL AND METHODS
Collection of Plant Sample
Fresh leaves of Costus afer were collected for the study. The collection site of the plant was Obio Akpa in Oruk Anam Local Government Area, Akwa Ibom State. The date of Collection was 15th November, 2023. The plants material was transported to University of Uyo, Uyo, Akwa Ibom State for identification and authentication of the plants. This was done at the Herbarium in the Department of Botany, University of Uyo, Uyo with herbarium voucher No: (Effiong, UUH 4420 (Uyo).
Preparation of Plant Material
After the identification, the leaves were washed and sun dried. The leaves were shredded and spread on cellophane and allowed to dry for 72 hours under room temperature. The dried leaves were pulverized (grinded) into fine powder using wooden pestle and mortar.
Preparation of Ethanolic Extract (Maceration and Extraction)
Cold extraction method (Maceration) was used in this research according to Hidayat and Wulandari (2021) [28], in the extraction procedure, 1000ml of 99% Concentrated Ethanol was used to Macerate 240g of the plant materials in an airtight container and kept in the laboratory under room temperature for 72 hours (3 days). The ethanolic suspension was filtered using a filter net and filter paper and the extract was evaporated in a water bath at 40° Celsius for 48 hours and stored in a beaker covered with aluminium foil for bioassay immediately after the evaporation was complete.
Collection of Test Organism
Fingerling of Clarias gariepinus were collected from Akwa Ibom State University fish farm, Obio Akpa Akwa Ibom State, Nigeria located within latitude 5°17′N and 7°27′N, Longitude 7°27′E and 7°58′E. The climate of the area is tropical and is characterized by distinct wet and dry seasons. The vegetation of the study area is generally rainforest close to the mangrove belt. Human activities in the area include farming, hunting, boat building and sand mining. A total of two hundred (200) fingerling were collected and used for the study.
Acclimatization of Specimen's
The fingerlings were acclimated in a re-circulatory plastic aquarium measuring 25 ×13 × 8.3 Cm3 containing hatchery water for 24hours in the fisheries and aquaculture laboratory of Akwa Ibom State fish farm. This enhanced the stability of the fingerlings from stress of collection and transportation (Udo et al, 2006).
Preparation of Experimental Aquaria
Ten (10) rectangular plastic aquaria measuring 25 ×13 × 8.3 Cm3 were thoroughly washed with tap water and properly rinsed with fresh water of similar salinity and allowed to drain dry for 24 hours on the laboratory bench based on Dede and Kagbo (2001).
Stocking of Specimen
Prior to commencement of actual experiment, a preliminary test or range finding test with varying concentration (0, 5, 10, 15, 20 mg/l) was conducted to give the actual variations in concentration to be used for the bioassay. Each of the aquarium had a replicate to ensure accuracy. Each of the Ten (10) plastic aquaria was filled with two liters of hatchery water and 10 Clarias gariepinus fingerlings was stocked in each aquarium. The ethanolic extract of Costus afer with varying concentrations (0,21, 22, 23, 24 mg/l) was added to each stocked aquaria and allowed to stand for 96 hours for mortality examination.
Monitoring of Water Quality
Water Quality Parameters was monitored prior to commencement of the experiment and also periodically according to Standard Method (APHA,1998) [29]. Parameters that were monitored include dissolve Oxygen (DO), pH, And Temperature (0C). Temperature and pH were measured using portable pH /Ec/ TDs/ Temperature HANNA, H1 991301 Model instrument while oxygen was measured using digital portable analyser JPB - 607A from "Search Tech Instrument".
Monitoring of Specimen for Mortality
The effects of the various concentration of the ethanolic extract of C. afer on the fingerlings was monitored on a 24 hours’ basis for 96 hours as recommended by Udo et. al., (2006) [30] and Ekanem and Ekpo (2008) [31].
Determination of Mortality and Survival Rates of Fingerlings
The percentage mortality and survival rates of the fingerlings in the different concentrations of the ethanolic extract of C. afer during the period of study was determine using the formula;
% mortality =n/N × 100 (Chan, 1977) [32].
Where;
n = number of dead fish per aquarium per concentration
N = Total Individual Stocked
The difference between dead fish and survivors will give the percentage survival of the fingerlings at the end of the experiment (96 hours) (Udo et. al., 2006) [30].
Determination of Mortality Lethal Median Concentration (96 hours LC50)
The effects of the various concentrations of the ethanolic extract of plant (C. afer) on the fingerlings of C. gariepinus was determined by graphical method (Probit Level Determination as recommended by Omoregie (2002) [33], Omoregie and Ufodike (2000) [34], Ekanem and Ekpo (2008) [31] and Udo et.al. (2006) [30]. At Lethal Median Concentration LC50, after 96 hours of test, the number of fingerlings that are expected to die was determined from the graph. Similarly, the concentration that will kill 50% of the stocked fingerlings at the end of the test (96 hours) was determined at the probit level (Omoregie, (2002) [33] Omoregie and Ufodike (2000) [34], Udo et. al., (2006) [30]; Ekanem and Ekpo (2008) [31].
Behavioral Assessment of the Test Organisms in the Toxicant
Behavioral morphological responses of C. gariepinus fingerling exposed to C. afer leaf extract were monitored and measured daily according to OECD, (2014) [34]. In addition to the acute doses, the control group without toxicant exposure were also observed as a guide for the assessment of any behavioral and morphological changes in the experimental groups. The responses monitored included irregular swimming, excessive mucus secretion, increased air gulping, vertical erection and respiratory distress Each experimental tank was observed for the concentrations and exposure durations
Collection of Samples for Histopathological Examinations
The gill's tissues were isolated from the test animal and fixed in formalin -saline for 48 hours. The fixed tissue was processed manually through graded ethanol, cleared in xylene impregnated and embedded in paraffin wax, sections of the tissue sample were cut with a rotary microtome, stained by hematoxylin and eosin technique, prepared tissues were finally observed using a microscope for pathological changes at x100 and x400 magnification.
Data Analysis
The results of the respective concentration effects of the ethanolic extract of C. afer was presented in tables. One-way analysis of variance (ANOVA) was used to test for significant difference between the varying concentrations in both batches (batch A and batch B) at the probability level of (P>0.05). Probit analysis was done using SPSS version 20.0.
RESULTS
Initial Water Quality Parameters
The initial water quality parameters prior to stocking are shown in Table 1. Dissolved oxygen had a value of 5.8 mg/l, with a value of 29.5oC for Temperature and 6.40 for pH.
Table 1: Initial Physico-chemical parameters of the test water prior to stocking of test organism.
|
Fish Species |
Initial physico-chemical parameters prior to stocking |
||
|
DO (mg/l) |
Temp (oC) |
pH |
|
|
Clarias gariepinus |
5.8 |
29.5 |
6.40 |
Variation in water Quality (Physico-chemical parameters) in the test media with Clarias gariepinus as test organism (Batches A and B) during the experimental period (96 Hours)
Table 2 shows the variation recorded in the different physico-chemical parameters for Clarias gariepinus (Batches A and B) in the different concentration of the extract and time.
Dissolved Oxygen (mg/l)
Dissolved oxygen concentrations ranged between 4.00 – 5.3 mg/l in the Omg/l concentration of the extract. The highest DO value was recorded at the 24 hours of test with the least value recorded during the 96 Hour of Test.
In the 21 mg/l concentration of toxicant, dissolved oxygen ranged between 2.8 – 4.8 mg/l. the least value was recorded during the 96 hours of test while the highest value was recorded during the 24 hours of test.
In the 22 and 23 mg/l concentration of toxicant, dissolved oxygen ranged 2.4 – 4.6 mg/l and 1.4 – 4.4 mg/l respectively. The highest and least value of DO were observe at the 24 hours of test and 96 hours of test for both concentrations during the study duration.
In the 5 mg/l concentration of toxicant, dissolved oxygen value ranged from 1.3 – 4.2 mg/l. The highest value was recorded during the 24 hour of test and the least value was observed during the 96 hours of test.
Temperature (oC)
Temperature value were observed to range between 25.1 – 27.6 oC during the 96 hours’ bioassay. The least value of 25.1 oC was recorded during the 72nd and 96th hours of test in the 24 mg/l concentration of toxicant while the highest value of 27.6 oC was recorded during the 24 hours of test in the 0 mg/l concentration of toxicant (control).
pH
The value of pH was observed to range between 5.80 – 6.31 during the 96 hours’ experimental bioassay. The least value of 5.80 was recorded during the 96th hours of test in the 22 mg/l concentration of toxicant while the highest value of 6.31was recorded during the 24 hour of test in the 0 mg/l concentration of toxicant (control).
Table 2: Summary of the variations in the physico-chemical parameters in the test media during the experimental period.
|
Parameters |
Conc. (mg/l) |
BATCH A |
BATCH B |
||||||
|
24 hrs. |
48 hrs. |
72 hrs. |
96 hrs. |
24 hrs. |
48 hrs. |
72 hrs. |
96 hrs. |
||
|
Dissolved Oxygen (mg/l) Initial: 5.8 mg/l |
O |
5.3 |
4.7 |
4.4 |
4.0 |
5.3 |
4.7 |
4.4 |
4.0 |
|
2 |
4.8 |
4.0 |
3.2 |
2.8 |
4.8 |
4.0 |
3.2 |
2.8 |
|
|
3 |
4.6 |
2.8 |
2.6 |
2.4 |
4.6 |
2.8 |
2.6 |
2.4 |
|
|
4 |
4.4 |
1..6 |
1.5 |
1.4 |
4.4 |
1..6 |
1.5 |
1.4 |
|
|
5 |
4.2 |
1..5 |
1.4 |
1.3 |
4.2 |
1..5 |
1.4 |
1.3 |
|
|
Temperature (oC) Initial: 29.5 oC |
O |
27.6 |
26.5 |
26.2 |
25.2 |
27.6 |
26.5 |
26.2 |
25.2 |
|
2 |
27.4 |
26.4 |
26.3 |
25.5 |
27.4 |
26.4 |
26.3 |
25.5 |
|
|
3 |
25.5 |
25.4 |
25.3 |
25.2 |
25.5 |
25.4 |
25.3 |
25.2 |
|
|
4 |
25.4 |
25.3 |
25.2 |
25.0 |
25.4 |
25.3 |
25.2 |
25.0 |
|
|
5 |
25.2 |
25.2 |
25.1 |
25.1 |
25.2 |
25.2 |
25.1 |
25.1 |
|
|
pH Initial: 6.40 |
O |
6.31 |
6.25 |
6.22 |
6.12 |
6.31 |
6.25 |
6.22 |
6.12 |
|
2 |
6.26 |
6.22 |
6.12 |
6.00 |
6.26 |
6.22 |
6.12 |
6.00 |
|
|
3 |
6.21 |
6.20 |
6.00 |
5.80 |
6.21 |
6.20 |
6.00 |
5.80 |
|
|
4 |
6.21 |
6.22 |
6.22 |
6.12 |
6.21 |
6.22 |
6.22 |
6.12 |
|
|
5 |
6.16 |
6.05 |
6.02 |
6.00 |
6.16 |
6.05 |
6.02 |
6.00 |
|
Summary of the Percentage Mortality and survivors of C. gariepinus Fingerlings in the different concentrations of the ethanoic extract of Costus afer at the end of the experiment (96 hrs.).
The percentage mortality and survivors of C. gariepinus fingerlings at the end of the test period in each of the concentrations are shown in Table 3 for the two batches of the experiment.
In the 0 mg/l concentration of the extract, no mortality was recorded throughout the test period in both batches A and B. in the 21 mg/l concentration of the extract, 70 % mortality was recorded leaving behind 30 % survivors in both bathes.
At the end of the 96-hour bioassay 100 % mortality was observed in the 22, 23 and 24 mg/l concentration of the extract leaving behind no test organisms in the test media for both batches (Table 3). Statistical Analysis using one-way Anova (SPSS 20.0) showed that there was no significant difference (p>0.05) in mortality between the two batches.
Table 3: Summary of the Percentage Mortality and survivors of C. gariepinus Fingerlings in the different concentrations of the ethanoic extract of Costus afer at the end of the experiment (96 hrs).
|
Conc. of extract (mg/l) |
BATCH A |
BATCH B |
||||||
|
|
Mortality (M) |
% M |
Survivors (S) |
% S |
Mortality (M) |
% M |
Survivors (S) |
% S |
|
0 |
0 |
0 |
10 |
100 |
0 |
0 |
10 |
100 |
|
21 |
7 |
70 |
3 |
30 |
7 |
70 |
3 |
30 |
|
22 |
10 |
100 |
0 |
0 |
10 |
100 |
0 |
0 |
|
23 |
10 |
100 |
0 |
0 |
10 |
100 |
0 |
0 |
|
24 |
10 |
100 |
0 |
0 |
10 |
100 |
0 |
0 |
96 Hours LC50 Determination
The 96 hours LC50 for C. gariepinus fingerlings exposed to the different concentrations of the ethanolic extract of Costus afer was determine using probit analysis. The concentrations were first transformed into log for the probit analysis (Table 4). The 96 hours LC50 is given at 20.5 mg/l representing a log transformed concentration of 1.31 mg/l a point where 50 % of the test organisms would be killed at the end of the experiment (Figure 1).
Table 4: LC50 determination for C. gariepinus Fingerlings at the end of the 96-hours bioassay.
|
Concentration (mg/l) |
Log Transformation |
Mortality (M) |
% Mortality |
Survivor (S) |
% Survivor |
|
0 |
0 |
0 |
0 |
0 |
100 |
|
21 |
1.32 |
10 |
100 |
3 |
30 |
|
22 |
1.34 |
10 |
100 |
0 |
0 |
|
23 |
1.36 |
10 |
100 |
0 |
0 |
|
24 |
1.38 |
10 |
100 |
0 |
0 |
Figure 1: Probit Graph of mortality (%) against concentration.
Behavioral Responses
Behavioral response of C. gariepinus fingerling exposed to concentrations of ethanolic extract of Costus afer leaf between 24 and 96-hours exposure durations were assessed via a semi-quantitative measurement (Table 5). After 24-h exposure, the treated groups exposed to 21–24 mg/l concentrations of the plant extract displayed slight to moderate behavioral changes such as excessive mucus secretion, air gulping, erratic swimming, respiratory distress and vertical erection, while the control and the lowest toxicant concentration exhibited none of these behavioral changes. Further exposure (48–96 hours.) to the plant leaf extract promoted moderate to severe earlier stated behavioral abnormalities in the treated groups, in a concentration-dependent pattern, except for 21 mg/l treated group.
Table 5: Effect of Costus afer leaf extract acute concentrations on behavior of Clarias gariepinus fingerlings at exposure duration intervals.
|
Time in hours |
Conc. (mg/l) |
Gulping of air |
Vertical erection |
Respiratory distress |
Erratic swimming |
Abnormal mucus secretion |
|
24 |
control |
- |
- |
- |
- |
- |
|
21 |
+ |
- |
- |
- |
- |
|
|
22 |
+ |
+ |
+ |
+ |
+ |
|
|
23 |
++ |
++ |
++ |
++ |
+ |
|
|
24 |
++ |
++ |
++ |
++ |
++ |
|
|
48 |
control |
- |
- |
- |
- |
- |
|
21 |
+ |
- |
- |
- |
- |
|
|
22 |
+ |
+ |
+ |
+ |
+ |
|
|
23 |
++ |
++ |
++ |
++ |
+ |
|
|
24 |
++ |
++ |
++ |
++ |
++ |
|
|
72 |
control |
- |
- |
- |
- |
- |
|
21 |
- |
- |
- |
- |
- |
|
|
22 |
+ |
+ |
+ |
+ |
+ |
|
|
23 |
++ |
++ |
++ |
++ |
+ |
|
|
24 |
+++ |
+++ |
+++ |
+++ |
++ |
|
|
96 |
control |
- |
- |
- |
- |
- |
|
21 |
- |
- |
- |
- |
- |
|
|
22 |
+ |
+ |
+ |
+ |
+ |
|
|
23 |
+++ |
+++ |
+++ |
+++ |
+++ |
|
|
24 |
+++ |
+++ |
+++ |
+++ |
+++ |
Key: - None (0%); + Mild (< 10%); ++ Moderate (10 – 50 %); +++ severe (> 50 %).
Histopathology of the gill of C. gariepinus Exposed to the different concentrations of the ethanolic extract of Costus afer
The histopathological findings as shown in Figure 1 depict distinct histological changes between the control group and experimental groups exposed to different concentrations of the ethanolic extract. Gills from the control group exhibited normal histological features, characterized by a well-organized gill arch structure. The primary lamella and secondary filament processes displayed high cellularity, and the centrally supporting cartilaginous epithelium was intact. The tissue was well-vascularized, with erythrocytes distributed throughout, indicative of normal vascular perfusion. No significant pathological changes were observed in specimens administered with 21mg/L of the ethanolic extract. Histological analysis revealed findings consistent with normal gill architecture, comparable to the control group.
In contrast, specimens exposed to higher concentrations of the ethanolic extract exhibited notable histological alterations. Diffuse epithelial degeneration of the gill arch, particularly affecting the lamellae, was evident across all experimental groups. Severe loss of epithelial cells was observed, suggesting extensive damage to the gill epithelium.
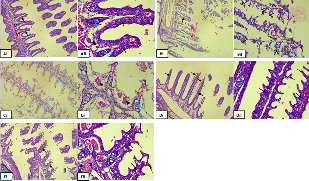 |
|
Figure 2: Photomicrograph of gills tissue section, A (i&ii) showing highly cellular Primary lamella and secondary filament at 0 mg/l of the extract, B(i&ii) gills tissue section showing highly cellular Primary lamella (thick arrow) and secondary filament (thin arrow) at 21 mg/l of the extract, C (i&ii), D (i&ii) and E (i&ii) showed degenerated gills epithelium (#) consisting predominantly the gill cartilage epithelial tissue at 22 mg/l, 23 mg/l and 24mg/l concentration of the extract respectively.
DISCUSSION
The present study investigated the toxic effect of ethanolic extract of costus afer leaves on the survival and histopathology of Clarias gariepinus fingerlings. The findings of our study confirmed the toxicity of C. afer leaf extract on C. gariepinus fingerling upon acute exposure. Prior to commencement of the experiment, basic physicochemical parameters were measured and ensured in conforms with the recommended standards for aquaculture operations. The values documented where in conformity with the approved or benchmark standards for save operations of aquaculture (Udo, 2007, Ajah 2007, George et.al., 2013a; George et.al., 2013b; George et.al., 2014a; George et.al., 2015a) [7,36-40].
The importance of this test is to ensure that it is not the presence of impurities or contaminants in the water that resulted in the toxic effects but rather the extract itself. Upon, introduction of the extract into the test media, variations in the test water were observed in the different concentrations and time of exposure. Theses variations could be attributed to the presence of bioactive compounds present in the extract, which influenced the overall toxicity observed in the experimental groups. Also, Variations in dissolved oxygen, temperature and pH levels of the test media could be attributed to the pollution potential of C. afer leaf extract. Presence of pollutants / toxicants in different forms can affect the living conditions and physico-chemical properties of an aquatic ecosystem (George, et. al., 2023 a) [41]. The growth and physiological processes of aquatic organisms is primarily dependent on good water quality (George, et. al., 2015a) [40]. Variations in water quality parameters of the test media have been reported in related studies by (George et.al., 2013a; George et.al., 2013b; George et.al., 2014a; George et.al., 2015a, George, et. al., 2015 c; George et. al., 2023a, b) [37-42].
In line with standard protocols or procedure in conducting toxicological studies, it is important to carry out a range findings or preliminary test before the actual experiment. The results of preliminary findings conducted showed that no mortality was recorded in the 0 mg/l (control) concentration of the extract used for the bioassay. Similarly, 100 % survivors were recorded in the 5, 10, 15 and 20 mg/l concentration of the extract with no mortality recorded. This provided a benchmark on safe concentrations to be administered for the final toxicity test.
The treatment groups which were exposed to the different doses of the extract of C. indica leaves exhibited mortality rates, with the exemption of the control group. This vindicates the toxic effects of cana indica on the fingerlings of C. gariepinus. However, the toxic effects of C. afer were more pronounced at higher concentrations of 22, 23 and 24 mg/l concentration of the extract were no fingerlings survived at the end of the 96-h bioassay. The control group justified the toxic effects of C. afer as no mortality was recorded throughout the 96-h duration. Effects of contaminants or toxicants being pronounced at higher concentrations has earlier been reported by (George et.al., 2013a; George et.al., 2013b; George et.al., 2015b; George et.al., 2014b; Essien-Ibok, 2020) [37,38,43,44].
In the present study, the toxic effects observed in the fish was directly proportional to the concentration of the C. indica extract to which they were exposed. This implies that as the concentration of the extract increases, the adverse effects on the fish become more noticeable resulting in mortality as was observed during the study. The results of the present findings is in consonance with earlier assertions reported by previous authors; (Ayuba et. al., 2002; Calta, et.al., 2004; Adedeji et.al., 2008; Ogundiran et.al., 2010) [45-48].
The 96-h median lethal concentration value (20.5 mg/l) representing a log transformed concentration of 1.31 mg/l obtained, indicates that C. indica leaf extract is severely toxic to the fish model. The present study to the best of our knowledge is the first to evaluate the toxicity of ethanolic extract of C. indica leaf to C. gariepinus fingerlings. Ayuba et al. (2002) [46] stated that Datura innoxia leaf extract at 120.23 mg/L caused median lethality in C. gariepinus fingerlings after 96 h acute exposure. Carica papaya seed powder at 12.9 mg/L, have been reported to produce median lethal effect in C. gariepinus fingerlings after 96-h exposure (Ayotunde et. al., 2010) [49]. Oribhabor and Akanse (2020) [50] reported a 96-h median lethal concentration of 0.65 mg/L Lanchocarpus cuanescans to C. gariepinus. Different plant extracts have varying levels of toxicity considering the plant parts and species, extraction methods, animal species and age (Okoro et. al., 2019; Ayuba et. al., 2012) [51,52].
Test organisms usually exhibit some behavioural changes as contaminants or toxicants is introduced into the test water. Behavioural abnormalities such as increased vertical swimming, erratic swimming, mucus secretion, respiratory distress and air gulping before death (Nwanna et. al., 2000) [53]. The pattern of behavioural changes observed in this study compared favourably with the report of (Fafioye et. al., 2004) [54] when African catfish (Clarias gariepinus) was exposed to Parkia biglobosa and Raphia vinefera extracts. Erratic swimming, air gulping, discoloration, loss of body equilibrium and mortality was also documented in Clarias gariepinus exposed to aqueous extracts of Blighiasapida and Kigelia africana (Onusiriuka and Ufodike, 1994) [55].
Histopathological examination of the gills is crucial for identifying structural alterations resulting from exposure to the ethanolic effects of Costus afer. The histological changes observed in the present study were concentration dependent with severe alteration been pronounced at higher concentration. Notable signs of pathological changes included disruption or loss of the gill epithelium integrity, pathological alterations in the primary and secondary gill lamellar and progressive degeneration of the gill tissues due to the exposure to the ethanolic extract of Costus afer. The results of this findings are similar to earlier assertion reported by George et. al., (2023, a, b, c, d) [40,56-58] when reporting on the acute toxic effects of Phramenthera capitata on the histomorphological changes in Clarias gariepinus and Oreochromis nioloticus fingerlings and Latana camara on the histological changes in Clarias gariepinus and Oreochromis nioloticus fingerlings respectively.
The pathological changes observed in the gill of C. gariepinus fingerlings exposed to the ethanolic extract of C. afer underscore the potential toxicity of this plant extract to aquatic organisms. Understanding these pathological alterations is essential for assessing the environmental impacts of C. afer and implementing appropriate mitigation measures to protect aquatic ecosystem.
SUMMARY AND CONCLUSION
Following the findings of this study, we infer that ethanolic extract of the leaves of C. afer adversely affected the behavior of C. gariepinus. In addition, the plant extract induced prominent histopathological lesions in the gill tissues of the exposed fishes and subsequently mortality. The indiscriminate application of the plant extract during fishing may cause harm to both targeted and non-targeted aquatic species when in contact with the plant substance.
RECOMMENDATIONS
Further toxicological studies to elucidate the dose-response relationship between C. afer exposure and pathological changes in the gills of C. gareipinus fingerlings are encouraged. Also, expanding the histopathological examinations to assess other vital organs such as the liver and kidney to comprehensively understand the systemic effects of C. afer extract exposure on C. gariepinus and other aquatic animals.
REFERENCES
- Dede EB, Kagbo HD. (2001). Aqua toxicological effects of water-soluble fraction (WSF) of diesel fuel on Oreochromis niloticus fingerlings. J Applied Science and Envir Manag. 5(1):93-96.
- Otoh AJ, Okoko AC, Ekanem IE, George UU, Asangusung PS. (2024 a). Effects of Multiple Batches of Stripped Eggs on the Reproductive Performances of Heterobranchus longifilis. Asian J Res Zool. 7(2):9-16.
- Otoh AJ, George UU, Asangusung PS, Okoko AC, Ekanem IE, Umaha MN. (2024 b). Investigation into the Impacts of Varied Batches of Stripped Eggs on the Reproductive Success of Clarias gariepinus. Asian J Res Zool. 7(2): 28-35.
- Otoh AJ, Ekanem IE, Okoko AC, Asangusung PS, George UU. (2024 c). Comparative Study of Inducing Broodstock with Natural and Artificial Hormones on Reproductive Performances of Clarias gariepinus. Asian J Fish Aquatic Res. 26(3): 31-38.
- Otoh AJ, Okoko AC, Asangusung PS, Ekanem IE, George UU, Idiong TE. (2024 d). Effect of Different Ages of African catfish (Clarias gariepinus) Broodstock on Reproductive Performance and Fries Production. Asian J Fish Aquatic Res. 26(3):39-47.
- Bala SA, Malachy NOA, Paul CO. (2015). Enzymatic and biochemical changes in common carp, Cyprinus carpio (L) fingerlings exposed to crude leaf extract of Cannabis sativa (L). Asian Pacific J Trop Dis. 5(2):107-115.
- Udo PJ. (2007). Techniques in Fish farming (Practice and Management). Wusen Publishers, Calabar, Nigeria:100.
- Nlewadim AA, Udoh JP, Otoh AJ. (2011). Growth response and survival of Heterobranchus Longifilis cultured at different water levels in outdoor concrete tanks. Aquaculture, Aquarium, Conservation & Legislation. 10(1):113-122.
- Nya E, Udosen I, Otoh A. (2017). Effect of Herbal based immunostimulant diets for disease control in African catfish Clarias gariepinus against Aeromonas hydrophia Infections. J Biol Agri Healthcare. 7(16):49-54.
- Otoh AJ, Udo MT, George UU. (2023a). Comparative Growth Performance and Sex Ratio of Heterobranchus longifilis and its Offspring Induced with Synthetic Hormone and Pituitary Gland of Heterobranchus longifilis. J Wetlands Waste Manag. 5(1):106-111.
- Otoh AJ, Udoh JP, Nya E, Asuquo IE. (2023b). Effect of Incremental Dilution of Catfish Sperm with Normal Saline Solution on Reproductive performance of Clarias gariepinus. J Wetlands Waste Manag. 5(1):74-78.
- Otoh AJ, Udo MT, George UU. (2022) Comparative Effect of Inducing Broodstock with Natural and Artificial Hormones on Reproductive Performances of Heterobranchus longifilis. Trop Freshwater Biol. 31: 95-102.
- Adedeji OB, Okocha RC. (2011). Constraint to aquaculture development in Nigeria and way forward. J Appl Sci Res. 7(7):1133-1140.
- Das SK, Mondal B, Biswas B, Mandal A. (2018). Herbal piscicides in inland aquaculture – a review. J Ecol Natural Resources. 2(3):1-8.
- Ekpo PB, Uno UU, Effiong EC, Etta SE. (2017). Acute toxicity of Tephrosia vogelii on early life stages of formed clarrid (Clarias gariepinus). Asian J Adv Agri Res. 3(2):1–5.
- Michael MA, George UU, Ekpo EA. (2015). Studies on the Physico-chemical Parameters of the Fresh Water Segment of the Lower Cross River System, South Eastern Nigeria. New York Sci J. 8(7):60-65.
- Ekpo EA, George UU, Edet M. (2015). Impacts of Water Pollution with Heavy Metal on the Tissue of Egeria radiata (Bivalvia: Tellinacea: Donacidae) (Lammark, 1804) Obtained from Calabar River, Cross River State, Nigeria. Nature Sci. 13(8):36-39.
- George UU, Etim IN, Ekanim MP, Akpan MK. (2015a). Acute Toxic Effects of Hevea brasiliensis on the Gills of Hatchery Reared Oreochromis niloticus Fingerlings. J Academia Industrial Res. 3 (11): 562-566.
- George UU, Etim IN, Ekanim MP, Out U. (2015b). Acute Toxicity of Ariel Detergent on the Survival of Fingerlings of Nile Tilapia (Oreochromis niloticus). Int J Current Res. 7(4):14825-14828.
- Jonah UE, George UU, Avoaja DA. (2020). Impacts of Agrochemical on Water Quality and Macroinvertebrates Abundance and Distribution in Ikpe Ikot Nkon River, South-South, Nigeria. Res. 12(1):36-43.
- George UU, Akpan ER, Akpan MM. (2020 a). Assessing the Impacts of Coastal Activities on the Water Quality of Qua Iboe River Estuary, South-South, Nigeria. New York Sci J. 13(3):1-15.
- George UU, Jonah UE, Nkpondion NN, Akpan MM. (2020 b). Assessment of Water Quality and Benthic Macroinvertebrates Assemblage of Etim Ekpo River, Niger Delta, Nigeria. World Rural Observation. 12(1):16–24.
- George UU, Edak E. (2018). Physical and Chemical Variations in Water Quality of Imo River Owing to Human Perturbations in the System. Researcher. 10(6):47–54.
- Jonah UE, George UU. (2019). Influence of Water Quality on Zooplankton Community Structure of Etim Ekpo River, Akwa Ibom State, South-South, Nigeria. World Rural Observation. 11(3):49-57.
- Jonah UE, George UU, Avoaja DA. (2019). Impacts of Anthropogenic Perturbation on Water Quality Characteristics of Ikpe Ikot Nkon River, Southern Nigeria. New York Sci J. 12(9):70-77.
- Okwu DE, Okwu ME. (2004). Chemical Composition of Spondias mombim Linn Plants parts. J Sustainable Agri Envir. 6:140-147.
- Anaga AO, Njoku CJ, Ekejiuba ES, Esiaka MN, Asuzu IU. (2004). Investigation of the methanolic leaf extract of Costus afer Ker for pharmacological activities in vitro and in vivo. Phytomedicine. 11:242-248.
- Hidayat R, Wulandari P. (2021). Methods of Extraction: Maceration, percolation and Decoction. Eureka Herba Indonesia. 2(1):68-74.
- APHA (American Public Health Association). (1998). Standard Methods for the examination of water and waste waters. 20th Edn. New York. 314.
- Udo PJ, Ekanem AP, Eze EE. (2006). Toxicity of crude oil to early life stages of Heterobranchus longifilis (Cruveier and Valiennces) Pisces: Bagridae). Trop Envir Res. 1:450-459.
- Ekanem AP, Ekpo IA. (2008). Effects of commercial detergents on the juvenile of Herterobranchus longifilis (Curvier and Valiennies). Afr J Envir Pollut Health. 6(1):18–23.
- Chan EI. (1977). Oil pollution and tropical littoral communities. Biological effects of the 1975 Floride key oiul spills. In: Procedures of oil spill Conference. Api publication, Washington DC. 187-192.
- Omoregie E. (2002). Acute Toxicity of Water-Soluble Fraction of Crude Oil to the Nile Tilapia (Oreochromis niloticus). Bull Envir Contamination Toxicity. 68:623–629.
- Omoregie E, Ufodike BC. (2000). Effects of water-soluble fraction of crude oil on growth of the Nile Tilapia (Oreochromis niloticus) (L). Bull Envir Contamination Toxicity. 64:601–605.
- Organisation of Economic Cooperation and Development (OECD). (2014). Guidelines for the Testing of Chemicals. Test Guidelines No.301, OECD, Paris, France.
- Ajah PO. (2007). Fish feeding and hatchery management. Calabar, Nigeria. Jerry Commercial Productions:178.
- George UU, Asuquo FE, Idung JU, Andem AB. (2013a). Effects of Lethal Concentration of Rubber Extract (Hevea brasiliensis) on the survival on fingerlings of Clarias gariepinus Under Laboratory Conditions. J Nat Sci Res. 3(9):56-60.
- George UU, Asuquo FE, Idung JU, Andem AB. (2013b). A Laboratory Bioassay of the Potential Effects of Rubber Extract (Hevea brasiliensis) on the survival of fingerlings of Oreochromis niloticus. J Biol Agri Health Care. 3(11):70-74.
- George UU, Joseph A, Andy JA. (2014a). Histopathological Alterations in Gills of Fingerlings of Clarias gariepinus (Burchell, 1822) Following Sublethal Acute Exposure to Hevea brasiliensis. Int J Sci Technol Res. 3(9):252- 255.
- George UU, Atakpa EO. (2015 a). Impacts of Human Perturbations on the Physico-chemistry and Biological Parameters on the Water Quality of Cross River Estuary, South Eastern Nigeria. Rep Opinion. 7(11):49-55.
- George UU, Ajayi OO, George IE, Vincent UE. (2023a). Establishing a Dose-Response Toxicity for Clarias gariepinus Fingerlings Exposed to Ethanolic Extract of Latana camara. Asia J Fisheries Aquatic Res. 24(1):1-10.
- George UU, Urom SE, Etanketuk N. (2014 b). Acute Toxic Effect of Qua Iboe Light Crude Oil on the Gills of Clarias gariepinus Juveniles. Int J Envir Pollut Res. 2(2):16-30.
- George UU, Atakpa EO. (2015b). Seasonal Variation in Physico-Chemical Characteristics of Cross River Estuary, South Eastern Nigeria. Nature Sci. 13(12):86-93.
- Essien-Ibok MA. (2020). The Toxicity of Ethanolic Extract of Alchornea cordifolia Leaf on Clarias gariepinus Fingerlings. Asian J Envir Ecol. 12(2): 22-28.
- Ayuba JO, Ofojekwu PC. (2002). Acute toxicity of the root of Jimson weed (Datura innoxia) to the African catfish (Clarias gariepinus) fingerlings. J Aquatic Sci. 17:131-133.
- Calta M, Ural MS. (2004). Acute toxicity of the synthetic pyrethroid deltamethrin to young mirror carp, Cyprinus carpio. Fresen Envir Bulletin. 13(11a):1179–1183.
- Adedeji BA, Adedeji AO, Adeyemo OK, Agbede SA. (2008). Acute toxicity of diaziam to the African catfish Clarias gariepinus. Afr J Biotechnol. 7(5):651-654.
- Ogundiran MA, Fawole OO, Aderoye SD, Ayandiran TA. (2010). Toxicological impacts of detergents effluent on juveniles of African catfish (Clarias gariepinus) (Burchell, 1822). Agri Biol J North America. 1(3):330–342.
- Ayotunde EO, Ofem BO, Okey IB, Ikpi GU, Ochang SN, Agbam NE, et al. (2010). Toxicity of pawpaw (Carica papaya) seed provide to sharp-tooth catfish (Clarias gariepinus) fingerlings and effects on haematological parameters. Int J Fisheries Aqua. 2:71-78.
- Oribhabor BJ, Akanse NN. (2020). Assessment of Ichthyotoxicity of Lonchocarpus cyanescens on the African catfish, Clarias gariepinus fingerlings and anuran tadpoles. Res J Envir Toxicol. 14:8–15.
- Ayuba VO, Ofojekwu PC, Musa SO. (2012). Acute toxicity of Clarias gariepinus exposed to Datura innoxia leaf extract. J Med Plants Res. 6 (12):2453–2457.
- Okoro N, Iheanacho SC, Nwakpa J, Eze K. (2019). Effects of Chromolaena odorata leaf extract on behaviour and haematology of Clarias gariepinus juveniles (Burchell, 1822). Afr J Aquat Sci. 44(4):421–427.
- Nwanna LC, Fagbenro OA, Ogunlowo ET. (2000). Toxicity of textile effluent to Clarias gariepinus and Heterobranchus bidorsalis fingerlings. Responsible aquaculture in the new millennium, Abstract of International Conference. (R. Flos and L) Cesswell Euro. Aquatic Society. Special Pub. No 28 Oostende Belgium. 510.
- Fafioye OO, Adebisi AA, Fagade SO. (2004). Toxicity of Parkia biglobosa and Raphia vimfera extracts on Clarias gariepinus juveniles. Afr J Biotechnol. 3(11):627-630.
- Onusiriuka BC, Ufodike FBC. (1994). Acute toxicity of water extracts of sausage plant and Akee Apple, Blighia sapida on African catfish, Clarias gariepinus. J Aquatic Sci. 9:35-41.
- George UU, Mbong EO, Abiaobo NO, Akpan II. (2023b). In Vivo Studies on Mortality and Histopathological Indices of Phragmenthera capitata (Mistletoes) on Clarias gariepinus Fingerlings in Aquarium. Mathews J Cytol Histol. 7(2):1-10.
- George UU, Otoh AJ, Ajayi OO, George IE. (2023c). Studies on mortality and Histopathological Alteration on the Gills of Oreochromis niloticus Juveniles Following Exposure to Ethanolic Extract of Phramenthera capitata Under Laboratory Conditions. Asian J Fish Aquatic Res. 24(3):23-34.
- George UU, Otoh AJ, Ajayi OO, George IE. (2023d). Dose-Response Relationship and Histo-Morphological Alterations on Oreochromis niloticus Juveniles following Exposure to Ethanolic Extract of Latana camara. Asian J Res Zool. 6(4):71-83.
.png)