Previous Issues Volume 9, Issue 8 - 2024
Evaluation of Bupivacaine Local Infiltration Effect with or Without Dexamethasone On Severity of Acute Pain in Women Undergoing Cesarean Section
Haleh Ayatollahi1, Farnaz Alizadeh Hooshyar2,*, Hadi Houshyar3, Samira Jahangard4,#
1Department of Obstetrics and Gynecology, School of Medicine, Solid Tumor Research Center, Urmia University of Medical Sciences, Urmia, Iran
2Department of Obstetrics and Gynecology, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
3Department of Anesthesiology, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
4Department of Obstetrics and Gynecology, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran
#Co-corresponding Author: Samira Jahangard, Department of Obstetrics and Gynecology, School of Medicine, Solid Tumor Research Center, Urmia University of Medical Sciences, Urmia, Iran, Tel: 00989105996264; Email: [email protected]
*Corresponding Author: Farnaz Alizadeh Hooshyar, Department of Obstetrics and Gynecology, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran, Email: [email protected]
Received Date: July 23, 2024
Published Date: September 18, 2024
Citation: Ayatollahi H, et al. (2024). Evaluation of Bupivacaine Local Infiltration Effect with or Without Dexamethasone On Severity of Acute Pain in Women Undergoing Cesarean Section. Mathews J Case Rep. 9(8):184.
Copyrights: Ayatollahi H, et al. (2024).
ABSTRACT
Introduction: The effectiveness of bupivacaine in reducing postsurgical pain has been a topic of debate in previous studies. Consequently, this study aimed to investigate the impact of dexamethasone and bupivacaine injection on pain intensity in patients undergoing cesarean surgery. Methods and Materials: In this double-blind randomized clinical trial, 75 pregnant women scheduled for cesarean section were divided into three groups, each consisting of 25 individuals. The first group received 0.5% bupivacaine in normal saline, the second group received 0.5% bupivacaine in normal saline along with dexamethasone, and the third group received an equivalent volume of normal saline as a control. Pain intensity was assessed using the Visual Analog Scale (VAS) at the time of entering recovery, one hour after surgery, and 24 hours after surgery. Findings: The administration of bupivacaine demonstrated superior pain control compared to the placebo, resulting in an average decrease of 2.88 points in the VAS pain score (P = 0.001, 95% CI = -1.81 to -3.95). Additionally, bupivacaine alone outperformed the combination of bupivacaine and dexamethasone, with an average reduction of 1.79 points in the VAS pain score (P = 0.001, 95% CI = -0.72 to -2.85). Conclusion: The findings of this study indicate that the use of bupivacaine can effectively reduce pain and the need for opioid painkillers in women undergoing cesarean section. However, the concurrent use of dexamethasone with bupivacaine diminishes its effectiveness to some extent. Our results indicate that the pains of three groups after injection of bupivacaine and bupivacaine/dexamethasone are in different levels. Also, the need for opioid painkillers in these three groups in order to reduce the pain are different.
Keywords: Acute Pain, Bupivacaine, Cesarean Section, Dexamethasone.
INTRODUCTION
Cesarean Delivery: Balancing Safety and Complications
Cesarean delivery, a common surgical intervention, plays a crucial role in safeguarding the lives of both mothers and infants. Despite efforts to promote natural childbirth, the utilization rate of this procedure has surged in recent years, maintaining an upward trend. In 2015, Cesarean deliveries accounted for 27% of all births in Europe and 32% in North America. Remarkably, Mehzad Urmia Hospital witnessed a substantial increase, with Cesarean deliveries constituting 52% of all births in 2021. However, this rise has raised concerns about potential complications for both maternal and fetal health.
Postoperative Pain and Its Implications
Following surgery, postoperative pain can lead to a cascade of adverse effects. These include heightened coagulability, compromised immune function, reactive hypoglycemia, delayed wound healing, increased myocardial oxygen consumption, paralytic ileus, and impaired respiratory function. These consequences stem from the activation of a neuroendocrine reflex and an elevation in sympathetic tone. Effective management of postoperative pain not only alleviates patient discomfort but also contributes to early mobilization, shorter hospital stays, reduced healthcare costs, and improved patient satisfaction. Furthermore, timely pain control after a caesarean section positively impacts early breastfeeding, aiding uterine contraction during the postpartum period.
Choosing the Right Pain Relief Approach
Selecting an appropriate pain relief method requires collaboration between the patient, obstetrician, and anesthesiologist. Key considerations include the patient’s anatomical and physiological characteristics, fetal condition, birth plan, and pharmacological properties of the medications employed. Various approaches exist for postoperative pain control, including systemic analgesia (both opioids and non-opioids) and local analgesic techniques (such as neuraxial and topical methods).
Optimizing Pain Management in Cesarean Sections: Balancing Analgesia and Safety
Narcotic analgesics, while highly effective in pain management, come with limitations due to adverse side effects such as nausea, pruritus, and ileus. In contrast, local analgesic methods offer distinct advantages over opioid and neuraxial approaches. They provide potent pain relief while minimizing side effects and reducing the risk of spinal hematoma.
Research on Topical Bupivacaine
Numerous studies have explored the efficacy of topical bupivacaine in mitigating post-cesarean pain, with most indicating favorable results. However, some studies have not observed significant differences in pain reduction when employing bupivacaine, particularly several hours after surgery. Consequently, further investigation in this area is warranted.
Combining Dexamethasone and Bupivacaine
Recent studies have revealed promising outcomes when co-administering dexamethasone, a long-acting corticosteroid, with bupivacaine. This combination enhances the effectiveness and duration of the analgesic effect. While evidence exists for other surgical contexts (such as vaginal prolapse surgery or supraclavicular nerve blocks), comprehensive research on combining dexamethasone with bupivacaine during cesarean sections remains scarce.
Study Objective
The present study aims to examine the effects of both sole bupivacaine injection and simultaneous injection of bupivacaine with dexamethasone at the surgical incision site on pain intensity in patients undergoing cesarean section.
MATERIALS AND METHODS
Study Design and Participants
A double-blind randomized clinical trial was conducted after obtaining approval from the ethics committee of Urmia University of Medical Sciences. The study included 75 pregnant women eligible for elective cesarean surgery. These sample size (75 pregnant women) under went to cesarean surgery as the period of 6 months.
Inclusion criteria comprised:
- Age between 18 and 40 years
- Classification as ASA 1 or ASA 2 based on physical condition
Exclusion criteria were as follows:
- Prolonged surgery duration
- Sensitivity to local anesthetics
- Previous multiple caesarean sections
- History of drug abuse
- Fibromyalgia or chronic nerve pain
- BMI over 35
- Changes in the surgical procedure
Randomization and Informed Consent
Randomization into three groups (25 individuals each) was accomplished using a random number table obtained from GraphPad. After obtaining informed consent, fundamental information such as age, medical history, previous pregnancies and deliveries, gestational age, drug or alcohol consumption, smoking habits, hookah usage, and patient weight was recorded.
Anesthesia and Intervention
All participants underwent spinal anesthesia with 12 mg of 0.5% bupivacaine. At the end of the surgery, the three groups received different injections:
- Group 1: 10 ml of 0.5% bupivacaine plus 10 ml of normal saline
- Group 2: 10 ml of 0.5% bupivacaine plus 8 ml of normal saline and 2 ml of dexamethasone
- Group 3: Normal saline injected into the fascia muscle and under the skin
The patients and injecting surgeon were blinded to the group assignment and injected drug.
Pain Assessment
Pain intensity was assessed using the Visual Analog Scale (VAS) at the following time points:
- Zero hour of entering recovery
- One hour after surgery
- Twenty-four hours after surgery
The conscious state of the patients, resulting from epidural or spinal anesthesia during the caesarean section rather than general anesthesia, facilitated their ability to respond to questions.
Patients with a VAS score equal to or greater than 4 received a 30 mg pethidine. Subsequently, they were admitted to the post-cesarean section unit. Based on the VAS, patients were prescribed 30 mg of pethidine every 6 hours and 100 mg of diclofenac suppository every 12 hours until the VAS dropped below 4. The initial administration time and total dose of pethidine received within the first 24 hours were recorded for the three groups and the results were compared. Furthermore, patients were observed for potential complications, and the relevant information was documented and compared. In our surgeries the complications have not been observed.
Statistical Analysis
Data analysis was performed using SPSS 22 software. The following steps were undertaken:
- Descriptive Statistics:
- Mean and variance were computed for quantitative variables.
- Number and percentage were reported for qualitative variables.
- Quantitative Variables:
- The assumption of normality was assessed.
- If the assumption was met, the relationship between variables was examined using Student’s t-test.
- In cases where the assumption of normality was not satisfied, the Mann-Whitney test was employed.
- Qualitative Variables:
- Chi-square test and Fisher’s exact test were utilized, if necessary, to analyze qualitative variables.
RESULTS
Patients Demographics and Characteristics
A total of 75 patients were enrolled in this study. The key demographic and clinical characteristics of the mothers are summarized below:
- Mean Age of Mothers: The average age of the mothers was 30.71 ± 6.49 years.
- Gestational Age: The average gestational age at the time of enrollment was 39.2 ± 0.6 weeks.
- Gravidity: These mothers had an average gravidity of 2 ± 1.
- Indications for Cesarean Section:
- In 57 cases (76%), the primary indication for cesarean section was a history of previous cesarean section.
- In 18 cases (24%), the cesarean section was performed due to breech presentation.
Postoperative Pain Intensity
Immediately after surgery, the postoperative pain intensity, as assessed by the Visual Analog Scale (VAS), had a median score of 1 (ranging from 0 to 9). This indicates that 81.3% of patients reported mild to no pain, while 18.7% experienced moderate to very severe pain (Table 1). At the one-hour mark post-surgery, the average VAS score increased to 6 (ranging from 0 to 10). During this period, 30.7% of patients reported mild or less pain, while 50.7% reported severe to very severe pain (Table 2). After 24 hours, the pain intensity, as measured by VAS, reached a median score of 4 (ranging from 0 to 8). Among the patients, 26.7% reported no to mild pain, while 32% experienced severe or very severe pain (Table 3).
Table1. Painintensityin patients based on VAS immediately after surgery
|
Number |
Percent |
Cumulative Percentage |
|
|
Nopain |
38 |
50.7 |
50.7 |
|
Mildpain |
23 |
30.7 |
81.3 |
|
Moderatepain |
8 |
10.7 |
92 |
|
Severepain |
1 |
1.3 |
93.3 |
|
Veryseverepain |
5 |
6.7 |
100 |
|
Total |
75 |
100 |
|
|
Number |
Percent |
Cumulative Percentage |
|
|
Nopain |
7 |
9.3 |
9.3 |
|
mildpain |
16 |
21.3 |
30.7 |
|
moderatepain |
14 |
18.7 |
49.3 |
|
Severepain |
15 |
20 |
69.3 |
|
Veryseverepain |
16 |
21.3 |
70.7 |
|
Theworst possible pain |
7 |
9.3 |
100 |
|
Total |
75 |
100 |
|
|
Number |
Percent |
Cumulative Percentage |
|
|
Nopain |
3 |
4 |
4 |
|
mildpain |
17 |
22.7 |
26.7 |
|
moderatepain |
31 |
41.3 |
68 |
|
Severepain |
22 |
29.3 |
97.3 |
|
Veryseverepain |
2 |
2.7 |
100 |
|
Total |
75 |
100 |
|
Study Results by Treatment Groups: The study participants were divided into three groups, each consisting of 25 individuals, with a balanced ratio of 1:1:1. The average age of mothers in the control group was 31.64 ± 6.14 years, in the bupivacaine + dexamethasone group it was 29.28 ± 6.14 years, and in the bupivacaine group it was 31.26 ± 7.21 years. There was no significant difference in age between the groups (P = 0.393). However, when examining gestational age, the control group had an average of 39.41 ± 0.73 weeks, the bupivacaine + dexamethasone group had an average of 39.08 ± 0.28 weeks, and the bupivacaine group had an average of 39.26 ± 0.69 weeks. A significant difference was not observed among the groups in terms of gestational age (P = 0.739).
The mean gravidity for the control group was 2.6 ± 0.1, for the bupivacaine + dexamethasone group it was 2.4 ± 12.4, and for the bupivacaine group it was 2.4 ± 1.2. There was no significant difference in gravidity between the groups (P = 0.524)
Postoperative Pain Levels Based on VAS: Immediately after the operation, the pain levels, as assessed by the Visual Analog Scale (VAS), were 2 ± 2 in the control group, 3 ± 3 in the bupivacaine + dexamethasone group, and 0 ± 1 in the bupivacaine group. Significant differences in pain levels were observed among the three groups at this time point (P = 0.002). One-hour post-surgery, pain levels increased to 8 ± 2 in the control group, 5 ± 3 in the bupivacaine + dexamethasone group, and 3 ± 3 in the bupivacaine group. Again, significant differences were noted among the three groups (P = 0.1). After 24 hours, the pain intensity, according to VAS, was 6 ± 1 in the control group, 4 ± 2 in the bupivacaine + dexamethasone group, and 3 ± 2 in the bupivacaine group. Once more, significant differences were found among the groups (P = 0.001) (Table 4).
Table 4. Classification of pain level in three groups based on VAS
|
Time |
intensity of pain |
groups |
|||||
|
control group |
bupivacaine+ Dexamethasone |
bupivacaine |
|||||
|
Number |
Percent |
Number |
Percent |
Number |
Percent |
||
|
Immediately after the surgery |
Nopain |
10 |
40.0% |
8 |
32.0% |
20 |
80.0% |
|
mildpain |
11 |
44.0% |
8 |
32.0% |
4 |
16.0% |
|
|
moderatepain |
2 |
8.0% |
5 |
20.0% |
1 |
4.0% |
|
|
Severepain |
1 |
4.0% |
0 |
0.0% |
0 |
0.0% |
|
|
Veryseverepain |
1 |
4.0% |
4 |
16.0% |
0 |
0.0% |
|
|
worstpain |
0 |
0.0% |
0 |
0.0% |
0 |
0.0% |
|
|
One hour after the surgery |
Nopain |
0 |
0.0% |
0 |
0.0% |
7 |
28.0% |
|
mildpain |
0 |
0.0% |
9 |
36.0% |
7 |
28.0% |
|
|
moderatepain |
3 |
12.0% |
4 |
16.0% |
7 |
28.0% |
|
|
Severepain |
5 |
20.0% |
8 |
32.0% |
2 |
8.0% |
|
|
Veryseverepain |
12 |
48.0% |
2 |
8.0% |
2 |
8.0% |
|
|
worstpain |
5 |
20.0% |
2 |
8.0% |
0 |
0.0% |
|
|
24 hours after the surgery |
Nopain |
0 |
0.0% |
3 |
12.0% |
0 |
0.0% |
|
mildpain |
0 |
0.0% |
4 |
16.0% |
13 |
52.0% |
|
|
moderatepain |
9 |
36.0% |
12 |
48.0% |
10 |
40.0% |
|
|
Severepain |
14 |
56.0% |
6 |
24.0% |
2 |
8.0% |
|
|
Veryseverepain |
2 |
8.0% |
0 |
0.0% |
0 |
0.0% |
|
|
worstpain |
0 |
0.0% |
0 |
0.0% |
0 |
0.0% |
|
When comparing the groups at different time points, it was observed that treatment with bupivacaine resulted in a significant reduction in VAS pain scores compared to the control group, with an average decrease of 2.88 points (p = 0.001, 95% CI = -1.81 to -3.95). Additionally, bupivacaine alone outperformed the combination of bupivacaine + dexamethasone, with an average reduction of 1.79 points in VAS pain scores (P = 0.001, 95% CI = -0.72 to -2.85) (Table5).
Table 5. Pain level in three groups based on VAS
Analgesic Administration:
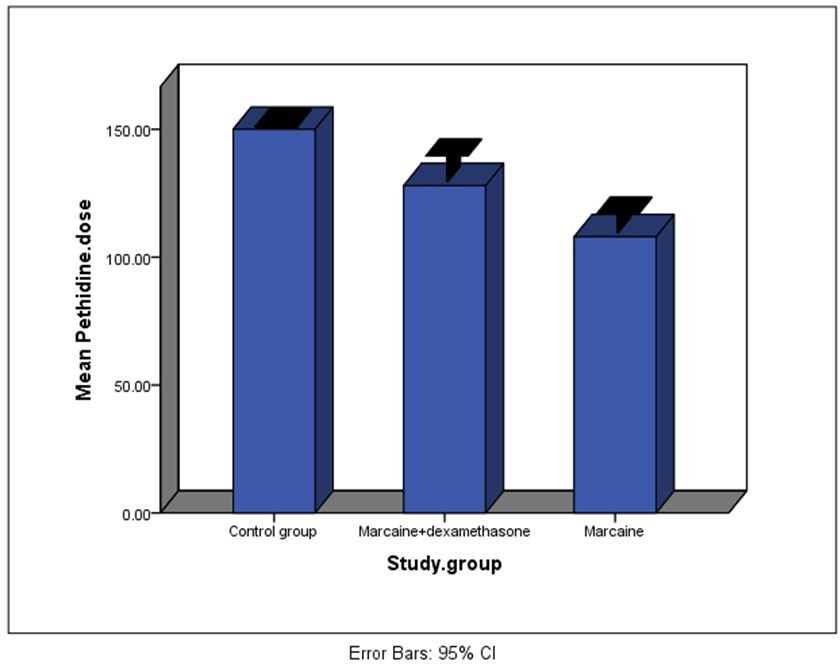
- The average dose of pethidine administered was 150 ± 0.0 mg in the control group, 108 ± 18.7 mg in the bupivacaine group, and 128 ± 25.3 mg in the bupivacaine + dexamethasone group. The three groups exhibited a significant difference in terms of pethidine dosage (P = 0.001) (Chart 1).
Chart 1. Chart of pethidine dosage in 24hours after surgery in three groups.
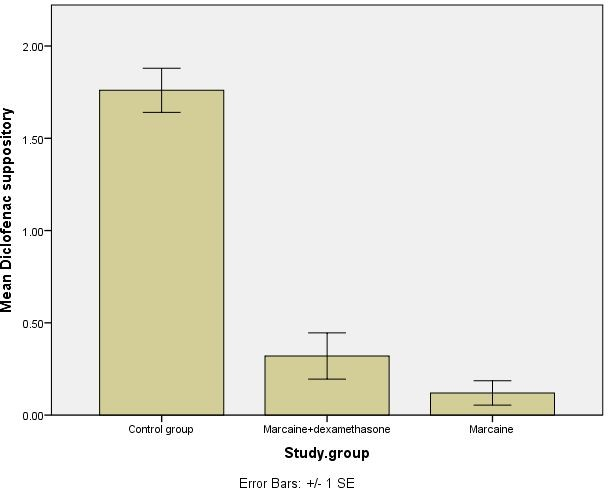
- The number of diclofenac suppositories used was 1.7 ± 0.6 in the control group, 0.12 ± 0.33 in the bupivacaine group, and 0.32 ± 0.63 in the bupivacaine + dexamethasone group. Both intervention groups showed significantly fewer diclofenac suppository administrations compared to the control group (P < 0.001). However, there was no significant difference between the two intervention groups (P = 0.233) (Table 6 and Chart2).
Chart 2. Diagram of the number of diclofenac suppositories consumed within 24 hours after the surgery.
Table 6. Use of non-opioid pain killers
|
control group |
bupivacaine+ Dexamethasone |
bupivacaine |
|||||
|
Number |
Percent |
Number |
Percent |
Number |
Percent |
||
|
The number of timesdiclofenac uses |
0 |
2 |
8.0% |
19 |
76.0% |
22 |
88.0% |
|
1 |
2 |
8.0% |
4 |
16.0% |
3 |
12.0% |
|
|
2 |
21 |
84.0% |
2 |
8.0% |
0 |
0.0% |
|
DISCUSSION
The utilization of cesarean delivery has seen a significant increase in recent years, despite efforts to promote vaginal birth and reduce unnecessary cesarean procedures. Effective pain management following cesarean surgery is crucial for the well-being of mothers. Therefore, selecting appropriate medications plays a paramount role in this context. This study aimed to investigate the impact of bupivacaine injection at the surgical incision site on pain intensity in patients undergoing cesarean section. Additionally, previous research has suggested enhanced analgesic properties when bupivacaine is administered concurrently with dexamethasone. Thus, this study also explored the simultaneous use of dexamethasone and bupivacaine in a distinct group.
The results of this double-blind clinical trial revealed that bupivacaine significantly reduced patient pain and the need for both opioid and non-opioid analgesics. Notably, the bupivacaine group had significantly fewer patients experiencing severe pain within the first 24 hours compared to the control group. However, the effectiveness of bupivacaine was diminished when used alongside dexamethasone. This finding aligns with certain previous studies. For example, Tavakol et al. conducted a clinical trial investigating the effect of subcutaneous bupivacaine injection in reducing pain after cesarean section. In their study, 102 pregnant candidates for cesarean delivery were randomly assigned to equal intervention and control groups. The intervention group received a subcutaneous injection of 10 ml of 0.5% bupivacaine mixed with 10 ml of distilled water at the incision site, while the control group received a 20 ml injection of distilled water.
Consistent with the present investigation, a previous study demonstrated that subcutaneous bupivacaine injection is a desirable and appropriate method for alleviating post-cesarean pain. The average pain scores at various time intervals in the intervention and control groups were 1.72 and 3.85, respectively. Furthermore, the average consumption of diclofenac suppositories in the ward was 0.35 in the intervention group and 1.5 in the control group, with a significant difference between the two groups (P < 0.05).
Additionally, Ghanaei et al. conducted a double-blind clinical trial in Gilan to compare the effect of injecting 0.25% bupivacaine with 2%. Parallel to the present study, their research demonstrated that local injection of 0.25% bupivacaine at the incision site during cesarean surgery effectively reduces pain intensity in the early postoperative period and diminishes the need for painkillers. Similarly, Niklasson et al. conducted a randomized clinical trial to examine the impact of bupivacaine-adrenaline injection near the fascia during surgery on pain reduction following cesarean section. Their findings, consistent with the current investigation, showed that administering bupivacaine with adrenaline into the surgical wound significantly decreases the requirement for morphine during the initial 12 hours post-cesarean section and effectively manages pain in women undergoing the procedure. Specifically, the average amount of morphine needed within the first 12 hours after surgery was 0.19 mg in the case group, compared to 0.24 mg in the control group, with a statistically significant difference observed only within the first 6 hours (P < 0.05).
The present study aimed to investigate the simultaneous effects of co-administering bupivacaine with dexamethasone. The results demonstrated that the concomitant use of dexamethasone attenuated the beneficial and positive effects of bupivacaine. Specifically, the amount of pain reported on the Visual Analog Scale (VAS) and the consumption of opioid and non-opioid analgesics within 24 hours post-surgery was significantly higher in the group receiving the dexamethasone and bupivacaine co-injection compared to the group receiving bupivacaine alone. These findings suggest that co-administration of above mentioned drugs may lead to drug dilution and delay in onset and peak effects.
Several studies have examined the impact of co-administration of bupivacaine and dexamethasone, yielding inconsistent findings. Consistent with the present study’s outcomes, Giugale et al. conducted a study to examine whether preoperative pelvic floor muscle injection and pudendal nerve blocks with bupivacaine and dexamethasone contribute to postoperative pain reduction following vaginal tissue prolapse repair when compared to saline and bupivacaine. Their findings indicated that preoperative pelvic floor muscle injections and pudendal nerve blocks with bupivacaine and dexamethasone did not improve postoperative pain after vaginal native tissue prolapse procedures.
In summary, the present study demonstrated the significant efficacy of bupivacaine in pain management and the reduction of analgesic requirements among mothers undergoing cesarean delivery, with no reported specific adverse effects in the patients. The utilization of bupivacaine for pain control can effectively alleviate patient discomfort following surgery. Although the current study did not investigate the implications of improved pain control, it is plausible that such an outcome could lead to various benefits, including enhanced ambulation, reduced hospitalization duration, decreased healthcare costs, and increased patient satisfaction. Furthermore, adequate and prompt pain management following cesarean section positively influences early breastfeeding, which, in turn, aids uterine contraction and involution during the postpartum period [1-15].
CONCLUSION
The findings of the current study, designed as a double-blind clinical trial, revealed that the administration of bupivacaine resulted in a significant reduction in patient pain and the necessity for both opioid and non-opioid analgesics. Notably, the number of patients experiencing severe pain within the first 24 hours was significantly lower in the bupivacaine group compared to the control group. However, when dexamethasone was combined with bupivacaine, its effectiveness was significantly diminished.
CONFLICT OF INTEREST
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
CLINICA LTRIAL IDENTIFICATION NUMBER
IRCT20220501054713N1 URL of the registration site: https://en.irct.ir/trial/63347
ETHICS BOARD APPROVAL NUMBER
IR.UMSU.REC.1401.036
URL of the registration site: https://ethics.research.ac.ir/ProposalCertificateEn.php?id=258599&Print=true&NoPrintHeader=t rue&NoPrintFooter=true&NoPrintPageBorder=true&LetterPrint=true
REFERENCE
- Grobman WA, Sandoval G, Rice MM, Bailit JL, Chauhan SP, Costantine MM, et al. (2021). Prediction of vaginal birth after cesarean delivery in term gestations: a calculator without race and ethnicity. Am J Obstet Gynecol. 225(6):664.e1-664.e7.
- Asıcıoglu O, Güngördük K, Yildirim G, Asıcıoglu BB, Güngördük OÇ, Ark C, et al. (2014). Second-stage vs first-stage caesarean delivery: comparison of maternal and perinatal outcomes. J Obstet Gynaecol. 34(7):598-604.
- Pirie K, Traer E, Finniss D, Myles PS, Riedel B. (2022). Currentapproachestoacutepostoperativepainmanagement after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 129(3):378-393.
- Bamigboye AA, Hofmeyr GJ. (2010). Caesarean section wound infiltration with local anaesthetic for postoperative pain relief - any benefit? South African Med J. 100(5):313-319.
- Usichenko TI, Klausenitz C, Hesse T, Cummings M. (2022). Effectiveness of acupuncture for pain control after Cesarean section is associated with the length of experience of the acupuncturist: observational data from a randomized clinical trial. Acupunct Med. 40(6):556-558.
- Carvalho B, Habib AS. (2019). Personalized analgesic management for cesarean delivery. Int J Obstet Anesth. 40:91-100.
- Arzola C, Wieczorek PM. (2011). Efficacy of low-dose bupivacaine in spinal anaesthesia for Caesarean delivery: Systematic review and meta-analysis. Br J Anaesth. 107(3):308-318.
- Tavakol K, Reiahinegad SH, Vahdaniati N. (2012). A Study on the Effect of Subcutaneous Injection of Bupivacaine (Marcaine) for Pain Relief after Cesarean. Armaghanedanesh.17(1):7-13.
- Niklasson B, Börjesson A, Carmnes UB, Segerdahl M, Öhman SG, Blanck A. (2012). Intraoperative injection of bupivacaine-adrenaline close to the fascia reduces morphine requirements after cesarean section: A randomized controlled trial. Acta Obstet Gynecol Scand. 91(12):1433-1439.
- Antoine C, Young BK. (2020). Cesarean section one hundred years 1920-2020: The Good, the Bad and the Ugly. J Perinat Med. 49(1):5-16.
- Herbert KA, Yurashevich M, Fuller M, Pedro CD, Habib AS. (2022). Impact of a multimodal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Neonatal Med. 35(24):4743-4749.
- Tabatabaei SMN, Rahat-Dahmarde A, Avval JO, Khazaie HA. (2022). Adding Dexamethasone To Intrathecal Bupivacaine 0.5%; Comparing the Anesthetic Ability With Bupivacaine 0.5% Alone Among Cesarean Section Patients. Medicni Perspekt. 27(2):82-88.
- Mansour Ghenaee M, Rahmani S, Jafarabadi M. (2015). Local lidocaine 2% in postoperative pain management in cesarean delivery. J Fam Reprod Heal. 9(1):19-21.
- GiugaleLE, Baranski LA, Meyn LA, Schott NJ, Emerick TD, Moalli PA. (2021). Preoperative Pelvic Floor Injections with Bupivacaine and Dexamethasone for Pain Control after Vaginal Prolapse Repair: A Randomized Controlled Trial. Obstet Gynecol. 137(1):21-31.
- Bindal D, Narang N, Mahindra R, Gupta H, Kubre J, Saxena A. (2018). Effect of dexamethasone on characteristics of supraclavicular nerve block with bupivacaine and ropivacaine: A prospective, double-blind, randomized control trial. Anesth Essays Res. 12(1):234.
.png)