Previous Issues Volume 4, Issue 1 - 2020
Determination of Levetiracetam in Plasma by a Modified HPLC-UV Method and Its Pharmacokinetic Application
Maria P. Hernandez-Mitre1, Susanna E. Medellin-Garibay1, Ildefonso Rodríguez-Leyva2, Cristian J. Rodríguez-Pinal1, Sergio Zarazúa1, Helgi H. Jung-Cook3, Suzanne L. Parker4, Silvia Romano-Moreno1, Rosa del C. Milán-Segovia1*
1Facultad de Ciencias Químicas, Universidad Autónoma de San Luis Potosí, S.L.P., México 2Hospital Central “Dr. Ignacio Morones Prieto”, San Luis Potosí, México 3Facultad de Química, Universidad Nacional Autónoma de México, Ciudad de México, México 4UQ Centre for Clinical Research, The University of Queensland, Brisbane, Australia
*Corresponding author: Rosa del Carmen Milan-Segovia, Laboratorio de Biofarmacia y Farmacocinética, Facultad de Ciencias Químicas de la Universidad Autónoma de San Luis Potosí, Av. Dr. Manuel Nava 6, Zona Universitaria. CP 78210, San Luis Potosí, México, Tel: +52-444-826-2300 (Ext. 6438); E-mail: [email protected]
Received Date: September 30, 2020
Published Date: November 23, 2020
Citation: Hernández-Mitre MP, et al. (2020). Determination of levetiracetam in plasma by a modified HPLC-UV method and its pharmacokinetic application. Mathews J Pharm Sci. 4(1):04.
Copyright: Hernández-Mitre MP, et al. ©2020.
ABSTRACT
Levetiracetam is a very effective antiepileptic which has been pointed out as a drug that should be monitored, despite its almost ideal pharmacokinetics. The objective was to adapt an HPLC-UV method to quantify levetiracetam in plasma based on previously published techniques, to provide an affordable analysis for routine use in a low-source setting. Pretreatment of plasma samples consisted of a deproteinization with acetonitrile. The analytical conditions implemented were a mobile phase of potassium dihydrogen phosphate buffer (10 mM, pH-4.6)/acetonitrile(93:7 v/v) at an isocratic flow rate of 0.5 mL/min, and UV detection at 192 nm. The retention time for levetiracetam was 7.45 min. Total runtime of the analysis was 9 min. The method was linear in the range of 5-60 µg/mL, repeatable and reproducible (CV<15%). Recovery was above 80%. The limit of detection was 0.19 µg/mL. The method was applied to determine levetiracetam concentration in plasma of 10 patients with epilepsy, and pharmacokinetic parameters Cmax, Tmax, AUC0-t, AUC0-∞, and t1/2 were estimated. The described method represents an alternative for laboratories interested in implementing therapeutic monitoring of levetiracetam that lack sophisticated equipment and have limited access to supplies employed in other published methods.
KEYWORDS: Levetiracetam; Epilepsy; HPLC-UV; Antiepileptic drugs; Therapeutic drug monitoring; Pharmacokinetics
INTRODUCTION
Levetiracetam is an antiepileptic drug (AED) used in patients of all age groups for the treatment of different types of seizures [1,2]. Despite its almost ideal pharmacokinetics, levetiracetam has been pointed out as an AED that should be monitored [3] due to its pronounced interindividual variation in response [4–6]. Therapeutic drug monitoring (TDM) guides physicians towards individualized plasma concentrations that are optimal for seizure control while minimizing toxic effects [6]. TDM of levetiracetam is performed in some centers around the world with a typical plasma concentration targeted between 12 and 46 µg/mL [3]. The objective of this study was to adapt an HPLC-UV method to quantify levetiracetam in plasma based on previously published techniques [7–14], to provide an affordable analysis for routine use in a low-source setting, directed to the therapeutic monitoring of levetiracetam in patients with epilepsy.
MATERIALS AND METHODS
Reagents and Standard Solutions
Sigma-Aldrich HPLC-grade levetiracetam (assay ≥98%), J.T. Baker® reagent grade monobasic and dibasic potassium phosphate, and Fermont HPLC-grade acetonitrile, methanol, and water were used. All solvents were filtered through a 0.45 µm nylon membrane filter before use. The primary stock solution was prepared at a concentration of 1 mg/mL of levetiracetam.
Apparatus and Chromatographic Conditions
The chromatographic analysis was performed using a Waters® HPLC system, equipped with an In-line Degasser-AF, a 1525 Binary Pump, a 717 Plus Autosampler and a 2487 Dual Absorbance Detector. Data was collected and analyzed using the software BREEZE™ (version 3.2). Compounds were separated using a reversed-phase Symmetry C18 column (3.5 µm particle size, 3.0mm x 150mm) protected by a Security Guard Symmetry C18 precolumn (5 µm particle size, 3.9mm x 20mm). The mobile phase was a mixture of potassium dihydrogen phosphate buffer (10 mM, pH 4.6) and acetonitrile (93:7 v/v) pumped at an isocratic flow rate of 0.5 mL/min. UV detection was performed at 192 nm.
Sample Preparation
100 µL of acetonitrile were added to 100 µL of plasma to deproteinize, vortexed for 1 min and then centrifuged at 20,817 g (14,000 rpm) for 20 min at 4°C. The supernatant was recovered and evaporated to dryness at 60°C using a Vacufuge® plus Concentrator. The dry residue was dissolved in 100 µL of mobile phase, of which 20 µL were injected into the chromatographic system.
Method Validation
The method validation was performed in agreement with the FDA guidelines [15].
Selectivity: Evaluated by demonstrating the non-interference of normal, lipemic and hemolyzed blank plasma, the use of heparin, citrate, and EDTA as anticoagulants, and antiepileptic drugs that patients could use in combination therapy with levetiracetam, such as gabapentin, carbamazepine, valproic acid, lamotrigine, and topiramate.
Linearity and Recovery: A set of calibration standards at concentrations of 5, 10, 20, 30, 40, 50 and 60 µg/mL was prepared by spiking pooled blank human plasma with the appropriate amount of levetiracetam solution, along with blank and zero samples. Calibration curves (n=3) were constructed by plotting the peak area of levetiracetam (y-axis) vs. levetiracetam concentration (x-axis). Linearity was assessed by determining the coefficient of correlation (r) of the points of the curves. The recovery of the analytes was evaluated using the calibration curve concentrations.
Lower Limit of Quantification (LLOQ), Upper Limit of Quantification (ULOQ) and Limit of Detection (LOD): The LLOQ was established as the lowest concentration standard of the calibration curve measured with acceptable precision (CV±20%) and accuracy (80-120%). The ULOQ was established as the highest concentration standard of the calibration curve measured with acceptable precision (CV±15%) and accuracy (85-115%). The LOD is the lowest amount of analyte which can be detected but not necessarily quantitated as an exact value, calculated based on the standard deviation of the blank.
Quality Control Samples (QCs): The QCs were prepared by supplementing blank plasma pools with the appropriate amounts of levetiracetam to yield concentrations of 15 (low QCs, LQCs), 35 (medium QCs, MQCs), 55 (high QCs, HQCs) and 200 µg/mL (diluted QCs, DQCs). A 1:4 dilution of the DQCs was carried out with blank plasma to achieve a concentration of 50 µg/mL. DQCs was used to demonstrate dilution integrity, used in case patients displayed plasma concentrations above the highest concentration of the calibration curve, since the dilution of samples should not affect the precision and accuracy [15]. Acceptance/rejection criteria for spiked, matrix-based calibration QCs were based on the nominal concentration of analytes.
Repeatability and Reproducibility: Precision was calculated as the coefficient of variation (%CV) of five measurements of each QCs in the same assay for repeatability and ten measurements of each QCs in a different assay for reproducibility. Accuracy was calculated as the degree of closeness of a determined value to the nominal value.
Stability: The stability of levetiracetam in plasma was investigated after three freeze-thaw cycles and 7, 14, 30, and 120 days at -80°C.
Pharmacokinetic Application
The study was approved by the Research and Ethics Committee of the Hospital Central “Dr. Ignacio Morones Prieto” (approval reference no. 86-16) and participants signed informed consent under the Declaration of Helsinki. Patients older than 18 years under treatment with immediate release levetiracetam for at least one week with the same dose, in monotherapy or combination therapy with other antiepileptic drugs were enrolled. Blood samples were collected in 3 mL sodium heparin tubes from an IV catheter attached to a CLC2000 connector right before oral administration, and at different times in the range from 15 min to 12 h after dose administration. Plasma was separated and stored at -80°C until analysis. Pharmacokinetic parameters Cmax, Tmax, AUC0-t, AUC0-∞, t1/2, CLss/F, Vz/F and MRT were estimated using non-compartmental analysis (NCA) with WinNonlin® Professional version 4.1 (Pharsight Corporation).
RESULTS
The retention time of levetiracetam was found to be 7.45 min. The total time of analysis was 9 min.
Method Validation
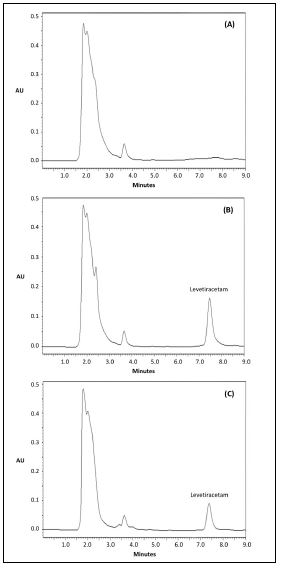
Selectivity: There was no interference of endogenous compounds of normal, lipemic and hemolyzed blank plasma. None of the anticoagulants tested interposed with the analytic peaks, or other antiepileptic drugs in combination therapy with levetiracetam. Chromatograms of blank plasma, plasma spiked with 60 µg/mL levetiracetam and plasma of a patient under treatment with 1500 mg of levetiracetam 12-hourly are shown in figure 1.
Figure 1: Chromatograms of (A) blank plasma, (B) blank plasma spiked with 60 µg/mL of levetiracetam, and (C) plasma of a patient under treatment with 1500 mg of levetiracetam 12-hourly.
Linearity and Recovery: Calibration curves (n=3) were linear in the range of 5–60 µg/mL. Equation of the regression line was y=128288x (± 318) + 190679 (± 54917) (r=0.999). The deviation of the calibration standards from the nominal concentrations was within ± 9% (˂15%). The overall recovery of levetiracetam was 91.0% ± 6.2 (CV=6.8%). Detailed results are presented in Table 1. The extent of recovery was consistent, precise, and reproducible at each concentration. Thus, the extraction process was efficient.
|
Nominal concentration (µg/mL) |
Mean observed concentration (µg/mL) ± SD |
Deviation from the nominal concentration (%) |
Recovery (%) ± SD |
|
5 |
4.6 ± 0.5 |
-8.4 |
102.0 ± 8.4 |
|
10 |
9.8 ± 0.5 |
-2.3 |
92.5 ± 2.8 |
|
20 |
20.4 ± 0.6 |
2.1 |
87.9 ± 5.0 |
|
30 |
30.6 ± 0.4 |
1.8 |
95.0 ± 4.0 |
|
40 |
40.5 ± 0.4 |
1.4 |
89.7 ± 2.2 |
|
50 |
49.0 ± 1.3 |
-2.0 |
83.3 ± 2.0 |
|
60 |
60.1 ± 0.3 |
0.2 |
86.9 ± 1.1 |
Table 1: Deviation of the calibration curve standards and recovery results (n=3). Deviation (%) = ([(mean observed concentration – nominal concentration)/nominal concentration] x 100; Recovery (%) = (response of analyte/response of standard) x 100.
Lower Limit of Quantification (LLOQ), Upper Limit of Quantification (ULOQ) and Limit of Detection (LOD): The LLOQ and ULOQ were set at 5 µg/mL and 60 µg/mL respectively, which cover the levetiracetam plasma concentration target of 12 to 46 µg/mL. The %CV corresponding to precision was 9.8% (± 20%) for LLOQ and 0.5% (± 15%) for ULOQ, while accuracy was found to be 91.6% (80-120%) for LLOQ and 100.2% (85-115%) for ULOQ. The calculated LOD was 0.19 µg/mL.
Repeatability and Reproducibility: Precision results are shown in Table 2. Both intra- and inter-day %CV were less than ±15% and accuracy was within 85-115%. Thus, the method is precise and accurate in terms of repeatability and reproducibility.
|
Nominal concentration (µg/mL) |
Repeatability (n = 5) |
Reproducibility (n = 10) |
||||
|
Mean observed concentration ± SD (µg/mL) |
% CV |
Accuracy (%) |
Mean observed concentration ± SD (µg/mL) |
% CV |
Accuracy (%) |
|
|
5 (LLOQ) |
4.4 ± 0.3 |
6.3 |
88.8 |
4.3 ± 0.5 |
10.8 |
85.5 |
|
15 (LQCs) |
14.8 ± 0.3 |
1.9 |
98.9 |
14.9 ± 0.7 |
4.4 |
99.0 |
|
35 (MQCs) |
35.2 ± 0.8 |
2.3 |
100.5 |
32.9 ± 2.4 |
7.2 |
94.1 |
|
55 (HQCs) |
54.7 ± 1.2 |
2.1 |
99.5 |
52.6 ± 2.4 |
4.6 |
95.7 |
|
200 (DQCs) |
196.9 ± 3.6 |
1.8 |
98.5 |
210.6 ± 14.9 |
7.1 |
105.3 |
Table 2: Precision and accuracy of the analysis of levetiracetam in human plasma. Precision (%CV) = (SD/mean observed concentration) x 100; Accuracy (%) = 100 ± [(mean observed concentration – nominal concentration)/nominal concentration] x 100.
Stability: Levetiracetam in plasma is stable (±15%) for at least three freeze-thaw cycles (± 7%) and after 7 (± 3.4%), 14 (± 3.0%), 30 (± 7.0%) and 120 days (± 4.2%) stored at -80°C.
Pharmacokinetic Application
Sixty-eight samples obtained from 10 patients with epilepsy aged between 22 and 85 years (4 elderly patients) receiving doses between 500 to 1500 mg 12-hourly were analyzed. The demographic characteristics of the studied population are summarized in Table 3. Since patients were under treatment with different levetiracetam doses, the dose-dependent parameters (Cmax, AUC0-t, and AUC0-∞) were normalized to 500 mg. The calculated pharmacokinetic parameters are also presented in Table 3. Mean dose normalized Cmax was 26.2 µg/mL (± 11.7), and Tmax was 1.0 h (± 0.5). The calculated value for t1/2 was 7.9 h (± 0.97) for young adults (˂ 60 years) and 9.7 h (± 2.7) for elderly adults (p = 0.177). CLss/F was 3.9 mL/h (± 2.0) for young adults and 3.0 mL/h (± 0.2) for elderly adults (p = 0.399). Four patients were in monotherapy with levetiracetam. Six patients were in combination therapy with other antiepileptic drugs such as gabapentin (1), carbamazepine (1), lamotrigine (1), valproic acid (2) and topiramate (2). This could explain part of the variability in reached levetiracetam plasma concentrations [16–18].
|
Characteristics |
Value |
|
Male patients |
40% |
|
Age (years) |
36.5 (22-85) |
|
Body weight (kg) |
67.9 ± 10.6 |
|
Height (m) |
1.65 ± 0.11 |
|
Serum creatinine |
0.8 (0.75-1.27) |
|
CrCL (mL/min) |
78.1 (53.5-159.7) |
|
Pharmacokinetic parameter |
Mean ± SD |
|
aCmax (µg/mL) |
26.2 ± 11.7 |
|
Tmax (h) |
1.0 ± 0.5 |
|
aAUC0-t (µg*h/mL) |
157.3 ± 52.0 |
|
aAUC0-∞ (µg*h/mL) |
262.5 ± 87.4 |
|
t1/2 (h) |
|
|
young adults (< 60 years) |
7.9 (± 0.97) |
|
elderly adults (≥ 60 years) |
9.7 (± 2.7) |
|
CLss/F (mL/h) |
|
|
young adults (< 60 years) |
3.9 /h (± 2.0) |
|
elderly adults (≥ 60 years) |
3.0 /h (± 0.2) |
|
Vz/F (mL) |
43.2 ± 19.3 |
|
MRT (h) |
12.5 ± 2.5 |
Table 3: Demographic characteristics of the studied population (n = 10) and obtained pharmacokinetic parameters of levetiracetam estimated by non-compartmental approach.
Data are shown as mean ± standard deviation or median (min-max). CrCL = creatinine clearance estimated using the Cockcroft-Gault equation. adose-dependent parameters normalized to 500 mg, Cmax = Maximum observed concentration, Tmax = Time to reach maximum observed concentration following drug administration, AUC0-t = Area under the plasma concentration-time curve from time zero to time t, AUC0-∞ = Area under the plasma concentration-time curve from time zero to infinity, t1/2 = Elimination half-life, CLss/F = Apparent total body clearance at steady state following oral administration, Vz/F = Apparent volume of distribution during terminal phase after oral administration, MRT = Mean residence time.
DISCUSSION
The present method offers an alternative to laboratories with limited access to reagents or solvents used in other methods. Techniques such as ultra HPLC-mass spectrometry have been developed; however, not all laboratories have such sophisticated equipment.
The LOD of 0.19 µg/mL obtained in the present method is higher than that reported by Martens-Lobenhoffer J, & Bode-Böger SM, [7] and Zufia L, et al. [13] of 0.1 and 0.1621 µg/mL respectively, but lower than that reported by Contin M, et al. [10] and Chhun S, et al. [11] of 1 µg/mL. In any case, it is not relevant to detect such low plasma concentrations that are below the lower limit of the plasma concentration target. The LLOQ of this method is the same as the method reported by Juenke JEM, et al. [8], which is enough to fulfill its purpose. Furthermore, it is selective for determining levetiracetam in combination therapy with other AEDs.
The use of expensive solid-phase [13,19] and liquid-liquid extraction with hazardous chemicals as hydrochloric acid [8] chloroform [8,11], ether [12] and dichloromethane [9,12,19] was averted. The use of perchloric acid to deproteinize [14] was also avoided. The deproteinization with methanol described by Contin M, et al. [10] was performed, but a pressure increase was observed in the chromatographic system after a small number of samples were injected. Thus, pretreatment with methanol was abandoned. A deproteinization with acetonitrile generated better results.
Good chromatographic separation was achieved when preparing the potassium dihydrogen phosphate buffer at a concentration of 10 mM instead of 50 mM [10], contributing to the use of less reagents. The flow rate of the mobile phase in the present method was lower than in other methods of 0.8 mL/min [9], 1 mL/min [7,12,19], 1.2 mL/min [13,14], and 1.5 mL/min [10]. Besides, other methods have longer runtimes of 10 [9], 14 [10], 17 [12] and 18 minutes [14]. The runtime of the analysis combined with a low flow rate translate into lower solvent expenditure.
The sample analysis in the present method can be performed using a small plasma volume of 100 µL, unlike other methods that require from 200 µL [7,9,12,13] to 500 µL of plasma [8,10,14]. Unlike other methods [7,8,12,13], an oven is not needed to maintain column temperature, since the analysis was performed at room temperature (25°C).
Limitations include that the total runtime of the analysis may be long for a single analyte, since it lacks an internal standard. However, the sample preparation is quite simple, and the chromatographic equipment is performing adequately; so, in this situation, an internal standard may not add any benefit.
The validated method was applied to quantitate levetiracetam in plasma of patients with epilepsy. The estimated pharmacokinetic parameters agree with reported values. According to Patsalos [2], with a dose of 500 mg of levetiracetam 12-hourly, the corresponding value for Cmax is 17 µg/mL (10–25); this value comes from multiple-dose studies, where participants were in monotherapy with levetiracetam. The data collected in the present study come from patients with epilepsy of different ages in monotherapy with levetira cetamor in combination therapy with other AEDs, which alter reached plasma concentrations by modifying levetiracetam clearance (CL) [16–18]. Combination therapy with enzyme-inducing AEDs (phenobarbital, phenytoin, primidone or carbamazepine) could increase levetiracetam CL, while concomitance with enzyme-inhibiting AEDs (valproic acid) could reduce levetiracetam CL [20]. In this case, Cmax values could vary according to the type of AED administered concomitantly. The estimated Tmax value also agree with reported values ranging from 0.6 to 1.3 h [1,2,4,21,22].
The reported t1/2 of levetiracetam is 6–8 h in young adults and 10-11 h in elderly adults [1,2,4,22]. The estimated values of t1/2 and CLss/F for elderly adults were higher than those obtained for younger adults. Longer t1/2 of levetiracetam is most likely due to an age-related decline in renal function. There is evidence of an age-related decrease in levetiracetam CLss/F in patients with epilepsy [23]. Although there is a trend, the sample size was too small to show statistically significant results in t1/2 and CLss/F values of young and elderly adults. Hence, TDM of levetiracetam is recommended in patients with renal impairment, in combination therapy with other AEDs, and in elderly adults with multiple co-morbidities under chronic treatment with different drugs, who have an increased likelihood of adverse effects and may require a dose adjustment.
CONCLUSION
An HPLC-UV method was adapted to quantify levetiracetam in plasma based on previously published techniques and validated in a range that covers the plasma concentration target of levetiracetam. The described method offers the possibility to perform the analysis with a small sample volume, a one-step deproteinizing procedure, and lower reagent and solvent expense while avoiding the use of hazardous chemicals.
ACKNOWLEDGMENTS
Authors would like to thank L.E. Herlinda Castillo Ibarra for her assistance during the blood sample collection procedure.
CONFLICTS OF INTEREST The authors declared no conflicts of interests.
FUNDING
María Patricia Hernández-Mitre had research support from CONACyT (scholarship number 438079). Susanna Edith Medellín-Garibay was promoted for Retention at Universidad Autónoma de San Luis Potosí through Support Program for the Incorporation of Scientists linked to the Institutional Consolidation of Research Groups and/or National Postgraduate Programs by CONACyT (Grant C-891/2018).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
REFERENCES
- Patsalos PN. (2000). Pharmacokinetic Profile of Levetiracetam: Toward Ideal Characteristics. Pharmacol Ther. 85:77–85. https://doi.org/http://dx.doi.org/10.1016/S0163-7258(99)00052-2.
- Patsalos PN. (2003). The Pharmacokinetic Characteristics of Levetiracetam. Methods Find Exp Clin Pharmacol. 25(2):123–129.
- Johannessen SI, Battino D, Berry DJ, Bialer M, Krämer G, et al. (2003). Therapeutic Drug Monitoring of the Newer Antiepileptic Drugs. Therapeutic Drug Monitoring. 347–363. https://doi.org/10.1097/00007691-200306000-00016.
- McMillin GA, Krasowski MD. (2016). Therapeutic Drug Monitoring of Newer Antiepileptic Drugs. Clin Challenges Ther Drug Monit Spec Popul Physiol Cond Pharmacogenomics. 25:101–134. https://doi.org/10.1016/B978-0-12-802025-8.00005-2.
- Gupta V, Gupta K, Singh G, Kaushal S. (2016). An Analytical Study to Correlate Serum Levels of Levetiracetam with Clinical Course in Patients with Epilepsy. J Neurosci Rural Pract. 7(1):S31–S36. https://doi.org/10.4103/0976-3147.196445.
- Krasowski MD. (2010). Therapeutic Drug Monitoring of the Newer Anti-Epilepsy Medications. Pharmaceuticals. 3:1909–1935. https://doi.org/10.3390/ph3061908.
- Martens-Lobenhoffer J, Bode-Böger SM. (2005). Determination of Levetiracetam in Human Plasma with Minimal Sample Pretreatment. J Chromatogr. 819:197–200. https://doi.org/10.1016/j.jchromb.2005.01.040.
- Juenke JEM, Brown PI, Urry FM, McMillin GA. (2006). Drug Monitoring and Toxicology: A Procedure for the Monitoring of Levetiracetam and Zonizamide by HPLC-UV. J Anal Toxicol. 30:27–30. https://doi.org/10.1093/chromsci/44.1.45.
- Lancelin F, Franchon E, Kraoul L, Garciau I, Brovedani S, et al. Therapeutic Drug Monitoring of Levetiracetam by High-Performance Liquid Chromatography with Photodiode Array Ultraviolet Detection: Preliminary Observations on Correlation between Plasma Concentration and Clinical Response in Patients with Refractory Epil. Ther Drug Monit. 29(5):576–583. https://doi.org/10.1097/FTD.0b013e318157032d.
- Contin M, Mohamed S, Albani F, Riva R, Baruzzi A. (2008). Simple and Validated HPLC-UV Analysis of Levetiracetam in Deproteinized Plasma of Patients with Epilepsy. J Chromatogr B Anal Technol Biomed Life Sci. 873:129–132. https://doi.org/10.1016/j.jchromb.2008.08.007.
- Chhun S, Jullien V, Rey E, Dulac O, Chiron C, et al. (2009). Population Pharmacokinetics of Levetiracetam and Dosing Recommendation in Children with Epilepsy. Epilepsia. 50(5):1150–1157. https://doi.org/10.1111/j.1528-1167.2008.01974.x.
- Ganesan M, Rauthan SK, Yadav SS, Pandey Y, Tripathi P. (2010). Validated Liquid Chromatographic Ultra Violet Method for the Quantization of Levetiracetam in Human Plasma Using Liquid-Liquid Extraction. Int J Pharm Sci Res. 1(1):1–9.
- Zufía L, Aldaz A, Ibáñez N, Giráldez J, Viteri C. (2010). LC Method for Therapeutic Drug Monitoring of Levetiracetam: Evaluation of the Assay Performance and Validation of Its Application in the Routine Area. Clin Biochem. 43:473–482. https://doi.org/10.1016/j.clinbiochem.2009.10.014.
- Sarmiento LA. (2014). Determinación de Levetiracetam En Suero Mediante HPLC-UV. Revista Med. 12–19.
- Food and Drug Administration. Bioanalytical Method Validation. Guidance for Industry. 2018.
- Pigeolet E, Jacqmin P, Sargentini-Maier ML, Stockis A. (2007). Population Pharmacokinetics of Levetiracetam in Japanese and Western Adults. Clin Pharmacokinet. 46(6):503–512. https://doi.org/10.2165/00003088-200746060-00004.
- Toublanc N, Sargentini-Maier ML, Lacroix B, Jacqmin P, Stockis A. (2008). Retrospective Population Pharmacokinetic Analysis of Levetiracetam in Children and Adolescents with Epilepsy. Dosing Recommendations. Clin. Pharmacokinet. 47(5):333–341. https://doi.org/10.2165/00003088-200847050-00004.
- Toublanc N, Lacroix BD, Yamamoto J. (2014). Development of an Integrated Population Pharmacokinetic Model for Oral Levetiracetam in Populations of Various Ages and Ethnicities. Drug Metab Pharmacokinet. 29(1):61–68. https://doi.org/10.2133/dmpk.DMPK-13-RG-045.
- Oláh E, Bacsói G, Fekete J, Sharma VK. (2012). Determination of Ng/ML Levetiracetam Using Ultra-High-Performance Liquid Chromatography-Photodiode Absorbance. J Chromatogr Sci. 50(3):253–258. https://doi.org/10.1093/chromsci/bmr053.
- Hirsch LJ, Arif H, Buchsbaum R, Weintraub D, Lee J, et al. (2007). Effect of Age and Comedication on Levetiracetam Pharmacokinetics and Tolerability. Epilepsia. 48(7):1351–1359. https://doi.org/10.1111/j.1528-1167.2007.01043.x.
- Perucca E, Johannessen SI. (2003). The Ideal Pharmacokinetic Properties of an Antiepileptic Drug: How Close Does Levetiracetam Come? Epileptic Disord. 5(1):17–26.
- Patsalos PN. (2004). Clinical Pharmacokinetics of Levetiracetam. Clin Pharmacokinet. 43(11):707–724. https://doi.org/10.2165/00003088-200443110-00002.
- Contin M, Mohamed S, Albani F, Riva R, Baruzzi A. (2012). Levetiracetam Clinical Pharmacokinetics in Elderly and Very Elderly Patients with Epilepsy. Epilepsy Res. 98:130–134. https://doi.org/10.1016/j.eplepsyres.2011.08.020.