Current Issue Volume 8, Issue 1 - 2024
BTXA/Prosigne and Redefinition of the Mandibular Contour - “Nefertiti Lift”: Academic Review
Ricardo Limongi Fernandes*
Dermatologist at the Federal University of São Paulo (UNIFESP). Specialist by the Brazilian Society of Dermatology (SBD) and International Fellow of the American Academy of Dermatology (AAD). Responsible for Medical Affairs at Cristália Injectables, Brazil
*Corresponding author: Dr. Ricardo Limongi Fernandes, Dermatologist at the Federal University of São Paulo (UNIFESP), Specialist by the Brazilian Society of Dermatology (SBD) and International Fellow of the American Academy of Dermatology (AAD), Responsible for Medical Affairs at Cristália Injectables, Brazil, Phone: +11 3085 3021, CRM-SP: 104.038 RQE 24.325, Email: [email protected]
Received Date: March 01, 2024
Published Date: March 15, 2024
Citation: Fernandes RL. (2024). BTXA/Prosigne and Redefinition of the Mandibular Contour - “Nefertiti Lift”: Academic Review. Mathews J Dermatol. 8(1):24.
Copyrights: Fernandes RL. © (2024).
ABSTRACT
Context: With the growing interest in treating the mandibular contour by a minimally invasive technique, botulinum toxin application in this area is increasing in popularity and relevance since the initial technique has been described. Objective: To present BTXA/Prosigne®, a brand marketed in Brazil since 2003, as an alternative to the toxins used in the literature involved in this indication, and to present an academic review on Nefertiti lift. Design: Academic review. Methodology: An electronic search of articles involving the use of botulinum toxin for redefinition of the mandibular contour was performed. Main anatomical aspects and techniques were highlighted. Conclusion: Many variations of the initial technique have been described, increasing the popularity and relevance of the procedure. It is particularly useful in younger patients with platysma muscle overactivity and good elastic skin containment. Although there is no consensus, a total of between 30 and 80U of Onabotulinumtoxin A seem sufficient for effective and safe treatment. The superficial injection technique is essential to guarantee safety and minimize the risk of adverse events. Clinical studies showing equivalence of efficacy and safety between BTXA/Prosigne® and Onabotulinumtoxin A. A dose equivalence of 1:1 suggests that we can reason with the same doses in the case of choosing BTXA/Prosigne®.
Keywords: Academic Review, Botulinum Toxin A, Mandibular Contour, BTXA, Prosigne, Nefertiti Lift.
INTRODUCTION
Developed as a medication in 1989 for the treatment of strabismus and blepharospasm associated with dystonia [1], botulinum toxin (BoNT) continues to accumulate indications for use. Currently, around 800 [2] are catalogued, including those contained in leaflets and off-label (although many of them lack more robust scientific evidence). Its great success began in 1992, with the first articles suggesting promising aesthetic effects [3] and, more quickly, after 2002 with the approval of this use in the United States by the FDA (Food and Drug Administration) and the publication of large clinical trials involving the treatment of dynamic wrinkles in the upper third of the face [4]. BoNT's growing success over the last 30 years is indisputable. Current data [5] show that it easily occupies the position of the most performed non-surgical aesthetic procedure globally, still with growth momentum of 17.7% compared to the previous year.
The aesthetic use of BoNT in the neck, similar to the objectives in the upper third of the face, was initially aimed at treating lines; However, some authors have already noticed some (until subjective) improvement in neck tension and jaw contour [6,7]. The use focused specifically on redefining the mandibular contour began with the development of the technique called 'Nefertiti Lift', in 2007. The technique promised to bring improvement in the definition of the edge and mandibular angle, being able to elevate the corners of the mouth and generate a 'mini lift' [8]. New variations and more recent modifications to the protocol have made the procedure gradually and progressively more popular [9].
The objectives of this article are: to present technical and historical concepts of botulinum toxin and BTXA/Prosigne® (Lanzhou Institute of Biological Products, Lanzhou, GS, China), presenting the latter as an alternative to the Abobotulinum, Incobotulinum and Onabotulinum Toxins used in the literature involved in this indication, present an academic review on the techniques involved in redefining the mandibular contour since the creation of the Nefertiti lift.
BOTULINUM TOXIN AND PROSIGNE®
Botulinum toxin is an exotoxin obtained from Clostridium botulinum cultures.
There are eight distinct types of botulinum toxin: A, B, C1, C2, D, E, F and G. All of them are serologically distinct from each other. Among them, type A is the one with the greatest toxicity and, therefore, the greatest clinical applicability.
Dr. Wang Yinchum, the creator of Botulinum Toxin A (BoNTA) marketed in Brazil since 2003 under the name Prosigne® and researcher at the Lanzhou Institute of Biological Products (LIBP), was born in 1937 and graduated in medicine from Qindao Medical School. More than 50 years of research have made him one of the foremost authorities on bacterial toxicology, most especially on Clostridium botulinum and botulinum toxin. Dr. Wang was the first to isolate type D and E toxins in China and was one of the developers of botulinum antitoxin, used in the emergency treatment of botulism.
In the 1980s, during the development of the first commercial preparation with BoNT produced from Hall A strains (Oculinum®), a project led by Dr. Scott, Dr. Wang's work and contributions were already included as a bibliographical reference in the Oculinum® literature. As a merit, Dr. Wang received samples of the Hall A strain as a donation in 1984 so that he could carry out his research on LIBP, which culminated in the development of a new product, named BTXA. In Brazil, BTXA is called Prosigne®, a Cristália Injectables brand.
Based on three years' production results and observation of phase III clinical studies organized by the China Medical Association in 10 hospitals, including Beijing Tongren Hospital, Beijing No.6 Hospital, Xian Central Hospital, Medical University Huaxi First Hospital, Ophthalmology Hospital Shanxi, Zhejiang Medical University Hospital, Hebei Hospital Zhangjiakou Fourth, Shandong Medical University Hospital, Lanzhou Medical College First Hospital and Shanghai Medical University Hospital Huashan in the People's Republic of China, with the participation of 500 patients, the BoNTA of LIBP was finally approved for commercial use as a new drug in December 1996. With advanced technology and first-world facilities, in 2001 LIBP was granted the Certificate of Good Manufacturing Practices (GMP). Since its commercial launch in 1997, BTXA/Prosigne® has been used in various parts of the world. Millions of patients have benefited from its treatments across a wide range of indications, including ophthalmology, neurology, physiatry, otolaryngology, gastroenterology, gynecology, urology, proctology, general surgery, orthopedics, plastic/craniofacial surgery and dermatology.
BTXA began to be exported in 2002. India and South Korea were the first two countries to receive this toxin. Currently, there are more than 30 countries with BTXA registration approved for sales, with different trade names, such as Dituroxal® (Mexico), Hengli® (China), Lantox® (Russia, Colombia and Kazakhstan), Lanzox® (Indonesia), BTXA® [formerly Liftox®] (Ecuador), Redux® (Peru) and Prosigne® (Brazil). BTXA/Prosigne® is still the only botulinum toxin approved by the Chinese health authority.
Post-marketing surveillance studies are underway in all countries to monitor the product's safety level, which has remained compatible with the most reputable products on the market [10].
The choice of LIBP (one of the world's largest producers of human albumin) to opt for a formulation containing sucrose, bovine gelatin and dextran in Prosigne® was not by chance. Gelatin had already been studied in the Oculinum® [1] project and, according to research conducted by Dr. Wang, with gelatin, the loss of toxicity is much lower during the freeze-drying process when compared to human albumin. This advantage is attributed to the absence of cysteine in bovine gelatin, an amino acid present in albumin and which may be related to the instability of the toxin. This means that the Prosigne® excipient is what guarantees greater stability to the tertiary structure of the toxin. Furthermore, it is the only excipient that allows obtaining prions free certification, an additional certificate of product safety against the risk of transmission of diseases caused by prions, not applicable to toxins containing human blood products.
Clinical studies in different indications have been carried out around the world and show efficacy and safety equivalent to that of Onabotulinum Toxin (and Aboboutlinum, respecting dose equivalence) [11,12,13,14,15], with which it shares many similarities, such as production from Hall A strains, its molecular weight of 900Kda and protein level of 5ng/100U [16]. The same studies suggest a 1:1 conversion rate between BTXA/Prosigne® and Onabotulinum Toxin.
NEFERTITI
Nefertiti [17] (1370-1330 BC) was a queen of the 18th dynasty of Ancient Egypt, main wife of Pharaoh Amenhotep IV, better known as Akhenáton. Her name means “the most beautiful has arrived”. Different features, such beauty and an unusual name in Egypt led many researchers to consider that Nefertiti had a foreign origin. However, in recent times the hypothesis that Nefertiti was Egyptian, daughter of Aí, a high-ranking Egyptian official responsible for the chariot corps who would become pharaoh after the death of Tutankhamun, has emerged.
On December 6, 1912, the famous bust of Queen Nefertiti was found in Amarna, sometimes also called the "Berlin bust" because it is located in the German capital. The discovery was the responsibility of an archaeological team from the German Oriental Society (Deutsche Orient Gesellchaft) led by Ludwig Borchardt (1863-1938). The piece was found in the residential area of the southern neighborhood of the city, in the house and workshop of the sculptor Tutemés. Until then, known representations of the queen showed her with an elongated skull, with the queen seen as a woman who supposedly suffered from tuberculosis. The bust proved to be decisive in changing the perception of the queen, one that many women of the 1930s would seek to imitate at masquerade balls.
In Archeology, the search for Nefertiti's mummy continues. In Dermatology, we continue to search for treatments to make the dreams of patients who want to have a mandibular contour like Nefertiti, the icon of beauty [18], come true (Figure 1).
Figure 1. Bust of Nefertiti.
APPLICATION TECHNIQUE
Nefertiti Lift is the attractive name given to a technique described by Levy in 2007 [8], whose objective is to redefine the mandibular line by neutralizing the downward movement of the platysma (Figure 2-8).
Figure 2. Movement of the platysma contributing to the appearance of ptosis of the lower third of the face.
The initially proposed technique was based on a study involving 130 patients with application of a line of injections performed along the mandibular margin, and another in the upper part of the posterior platysmal band, forming a “T”, using 15–20U of Onabotulinum Toxin in each side of the face. It was mentioned again by Gassia in 2009 [19], in the same way as it was initially described.
Figure 3. Mandibular and posterior platysmal band injection points [8].
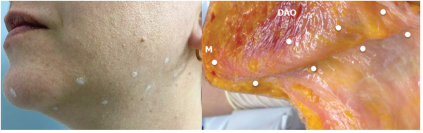
To assess whether or not patients are suitable for the Nefertiti Lift, the platysma muscle must be contracted so that the posterior vertical bands are clearly visible. Treatment is likely to be successful in patients when the mandibular border disappears with contraction of the platysma. The creator of the technique himself, Dr. Phillip M. Levy, in 2015 reaffirms the concepts of this use and suggests a double dose at the meeting point between the horizontal line (mandibular) and the vertical line (posterior platysmal band), as long as it is at least at least 1cm posterior to the hypothetical meeting point between the mandible and the nasolabial fold to prevent the toxin from being injected into the lower lip depressor muscle and reinforcing the maximum total dose of 20U of Onabotulinum Toxin per side [20].
In 2017, de Almeida [21] reinforced the usefulness of the protocol described by Levy, with the exception that the technique does not include the treatment of the anterior fibers of the platysma. She highlighted the importance of the upper portion of the platysma in the functional anatomy of the lower third of the face and how it can be targeted by myomodulators. To treat the lower third of the face as an anatomical unit, she suggests application to the mentalis muscle (intramuscularly), followed by superficial injections of BoNTA in a pattern consisting of 2 horizontal lines on each side of the lower face. The first line consists of 3 injection sites with 2U each, on the mandibular border, starting at the depressor anguli oris muscle and spaced 2 cm laterally, ending at the mandibular angle. The second horizontal line consisting of 4 points was placed 2 cm below the mandibular border, starting at an intermediate position between the m. mentalis and depressor anguli oris, every 2 cm, possibly with a slightly higher dose (2.5-3U) in the first two, running laterally to a point after the mandibular angle, using a total of 16–20 U per side. The protocol described by the authors includes the treatment of the three parts of the platysma (mandibular, labial and modiolar).
Figure 4. Injection pattern [21].
Still in 2017, Jabbour [22] evaluated 30 patients with a total average of 125U of Abobotulinum Toxin, using a protocol similar to that initially described for the Nefertiti lift. He found evident improvement in the platysmal bands, both at rest and during contraction; however, statistical analysis only indicated a trend towards improvement in the appearance of jowls, marionette lines and oral commissures. 1/3 of patients required touch-ups. Of these, half required treatment of the lower portion of the platysma due to the appearance of new bands that appeared after treatment of the upper platysma.
Awaida [23], in 2018, promoted a clinical trial with 25 patients (already submitted to the Nefertiti lift protocol 8 months previously) to evaluate the safety and effectiveness of the Microbotox [24] technique using validated scales and comparing them with the Nefertiti Lift. 140 to 210U of Abobotulinum Toxin were used in approximately 150 injections applied throughout the anterior region of the neck, in an area delimited superiorly by a line drawn 5cm above the mandibular border, medially by a vertical line 1cm posterior to the depressor angle of the mouth, posteriorly by the anterior border of the sternocleidomastoid muscle and inferiorly by the superior border of the clavicle. According to the results obtained, the authors suggest that the microbotox technique produces a firming effect on the skin, weakening the superficial fibers of the platysma. By paralyzing the superficial fibers of the platysma, it allows the skin to adapt to the underlying silhouette of the neck and lower face, improving jowl, neck volume and cervico-mandibular angle. On the other hand, platysmal bands at rest and contraction improved significantly more with the Nefertiti lift. This improvement was attributed to paralysis of the deep fibers of the platysma. The authors conclude that both techniques (Microbotox and Nefertiti) are effective and that specific patient complaints must be considered to establish a treatment plan with one, the other, or both techniques.
Figure 5. Application areas – Microbotox technique [22].
In 2022, Yi [24] highlights the anatomical aspects of BoNT injections into the platysma muscle. For the platysma band, the authors suggest a total of 40 U for 20 points on both sides of the medial and lateral bands, which should be injected intramuscularly (deeper injections) after palpation. For mandibular contouring, a total of 40U is injected into 20 points with subdermal injection (more superficial injections), in two lines: one above the lower edge of the mandible and the other below the line that connects the lip commissure with the earlobe.
Figure 6. Mandibular injection points and in the anterior and posterior platysmal bands [20].
Hernandez CA et al. [25] demonstrated that the “Toxin Lift” injection technique results in an increase in mid-facial volume, as well as a decrease in lower facial volume. The new injection algorithm presented may provide a safe and effective option for patients who wish to improve midfacial fullness and jaw contour with neuromodulatory treatment alone.
Figure 7. “Toxin lift” injection technique [25]. 4 points with 2 to 2.5U onabotulinum toxin (or equivalent) per point.
Germani M et al. [26] also propose that the “Toxin Lift” injection technique is capable of improving the clinical appearance of infraorbital cavities. The effects can be explained by the concepts of facial biomechanics. The authors highlight the high (although temporary, with resolution within 30 days) rates of adverse reactions (15.7%).
Figure 8. “Toxin lift” injection technique [26]. 4 points with 5U of abobotulinum toxin per point.
CONCLUSIONS
After 16 years of Levy's description of the technique, we can say that the Nefertiti lift was an important innovation, perhaps even ahead of its time, which may explain the little repercussion at that time [27]. With the growing interest in treating this region, variations of the initial technique have been described, increasing the popularity and relevance of the procedure. It is particularly useful in younger patients with platysma muscle over activity and good elastic skin containment. The test using platysma contraction, with attenuation of the jaw contour, is essential to predict the potential success of the treatment.
Although there is no consensus, a total of between 30 and 80U of Onabotulinum Toxin (or equivalent for Prosigne®), based on the technical specifications provided in the relevant references, are sufficient for effective and safe treatment. The superficial injection technique is essential to guarantee safety and minimize the risk of adverse events. Clinical studies showing equivalence of efficacy and safety between BTXA/Prosigne® and Onabotulinum Toxin with a dose equivalence of 1:1 suggest that we can reason with the same doses in the case of using BTXA/Prosigne®.
Like any aesthetic treatment, the combination of procedures is often essential to achieve optimized and natural results. Collagen biostimulation technologies, filling with hyaluronic acid, thread traction (or surgery) can complement the results.
Individual assessment, correct choice of material and appropriate application protocol can help patients achieve their dream of a Nefertiti facial contour.
REFERENCES
- Scott AB, Honeychurch D, Brin MF. Early development history of Botox (onabotulinumtoxinA). Medicine (Baltimore). 2023 Jul 1;102(S1):e32371.
- Botox: The Drug That’s Treating Everything. TIME Magazine January 16, 2017.
- Carruthers JD, Carruthers JA. (1992). Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 18(1):17-21.
- Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, et al. (2002). A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 46(6):840-849.
- International Society of Aesthetic Plastic Surgery (ISAPS). International Survey on Aesthetic/Cosmetic Procedures performed in 2021. United States: www.isaps.org.
- Brandt FS, Bellman B. (1998). Cosmetic use of botulinum A exotoxin for the aging neck. Dermatol Surg. 24(11):1232-1234.
- Kane MA. (1999). Nonsurgical treatment of platysmal bands with injection of botulinum toxin A. Plast Reconstr Surg. 103(2):656-663.
- Levy PM. (2007). The 'Nefertiti lift': a new technique for specific re-contouring of the jawline. J Cosmet Laser Ther. 9(4):249-252.
- Signorini M, Piero Fundarò S, Bertossi D, Cavallini M, Cirillo P, Natuzzi G, et al. (2022). OnabotulinumtoxinA from lines to facial reshaping: A new Italian consensus report. J Cosmet Dermatol. 21(2):550-563.
- Dados Farmacovigilância Cristália.
- Quagliato EM, Carelli EF, Viana MA. (2010). Prospective, randomized, double-blind study, comparing botulinum toxins type a botox and prosigne for blepharospasm and hemifacial spasm treatment. Clin Neuropharmacol. 33(1):27-31.
- Rieder CR, Schestatsky P, Socal MP, Monte TL, Fricke D, Costa J, et al. (2007). A double-blind, randomized, crossover study of prosigne versus botox in patients with blepharospasm and hemifacial spasm. Clin Neuropharmacol. 30(1):39-42.
- Ferreira LM, Talarico Filho S, Monteiro EO. (2009). Eficácia e tolerabilidade de uma nova toxina botulínica tipo A para tratamento estético das rugas faciais dinâmicas; estudo multicêntrico prospectivo de fase III. Surg Cosm Dermatol. 1(2):58-63.
- Morais OO, Reis-Filho EM, Pereira LV, Gomes CM, Alves G. (2012). Comparison of four botulinum neurotoxin type A preparations in the treatment of hyperdynamic forhead lines in men: A pilot study. J Dr Dermatol. 11(2):216-219.
- Costa A, et al. (2016). Estudo clínico multicêntrico, prospectivo, comparativo, randomizado e duplo cego, entre duas formulações de toxina botulínica tipo A registradas no Brasil para o tratamento das rugas da glabela. Surg Cosmet Dermatol. 8(1):33-40.
- He X, Miao CH, Li XJ, Wang YT, Liang Q, Zhang XP, et al. (2012). Biological characteristics and quality of botulinum toxin type A for injection. Chinese Journal of Biologicals. 25(11):1488-1491+1497.
- NEFERTITI. In: WIKIPÉDIA, a enciclopédia livre. Flórida: Wikimedia Foundation, 2023. Available at: https://pt.wikipedia.org/w/index.php?title=Nefertiti&oldid=65085145.
- Chauhan N. (2022). The Science Behind Nefertiti's Beauty: A Plastic Surgeon's Analysis. Indian J Plast Surg. 55(4):357-363.
- Gassia V, Beylot C, Béchaux S, Michaud T. (2009). Les techniques d'injection de la toxine botulique dans le tiers inférieur et moyen du visage, le cou et le décolleté. Le "Néfertiti lift" [Botulinum toxin injection techniques in the lower third and middle of the face, the neck and the décolleté: the "Nefertiti lift"]. Ann Dermatol Venereol. 136(Suppl 4):S111-S118.
- Levy PM. (2015). Neurotoxins: Current Concepts in Cosmetic Use on the Face and Neck--Jawline Contouring/Platysma Bands/Necklace Lines. Plast Reconstr Surg. 136(5 Suppl):80S-83S.
- de Almeida ART, Romiti A, Carruthers JDA. (2017). The Facial Platysma and Its Underappreciated Role in Lower Face Dynamics and Contour. Dermatol Surg. 43(8):1042-1049.
- Jabbour SF, Kechichian EG, Awaida CJ, Tomb RR, Nasr MW. (2017). Botulinum Toxin for Neck Rejuvenation: Assessing Efficacy and Redefining Patient Selection. Plast Reconstr Surg. 140(1):9e-17e.
- Cyril JA, Samer FJ, Youssef AR, Joseph SEK, Elio GK, Marwan WN. (2018). Evaluation of the Microbotox Technique: An Algorithmic Approach for Lower Face and Neck Rejuvenation and a Crossover Clinical Trial. Plast Reconstr Surg. 142(3):640-649.
- Yi KH, Lee JH, Lee K, Hu HW, Lee HJ, Kim HJ. (2022). Anatomical Proposal for Botulinum Neurotoxin Injection Targeting the Platysma Muscle for Treating Platysmal Band and Jawline Lifting: A Review. Toxins (Basel). 14(12):868.
- Hernandez CA, Davidovic K, Avelar LET, Alfertshofer M, Freytag DL, Frank K, et al. (2022). Facial Soft Tissue Repositioning With Neuromodulators: Lessons Learned From Facial Biomechanics. Aesthet Surg J. 42(10):1163-1171.
- Germani M, Almeida CCMS, Munoz-Lora VRM, Rogério V, Moelhoff N, Freytag DL, Green JB, et al. (2023). How to improve infraorbital hollows with neuromodulators-A clinical prospective interventional study about the application of facial biomechanics. J Cosmet Dermatol. 22(11):2950-2956.
- Signorini M, Piero Fundarò S, Bertossi D, Cavallini M, Cirillo P, Natuzzi G, et al. (2022). OnabotulinumtoxinA from lines to facial reshaping: A new Italian consensus report. J Cosmet Dermatol. 21(2):550-563.
.png)
.png)
.png)
.png)
.png)
.png)
.png)