Previous Issues Volume 9, Issue 10 - 2024
Atorvastatin-Induced Liver Injury with Rapid Enzyme Elevation: A Case-Report
Felipe Morales-León1,*, Cindy Sanhueza2, Pola Fernández-Rocca1, Tamara Sandoval-Quijada1, Elena María Vega1
1Clinical Pharmacy Division, School of Pharmacy, University of Concepción, Chile
2Pharmacy Service, Hospital Penco – Lirquén, Chile
*Corresponding Author: Felipe Morales-León, Pharm D. PhD. Associate Professor, School of Pharmacy, University of Concepción, Chile, Phone: 0056-41- 220 4208, Email: [email protected]
Received Date: October 17, 2024
Published Date: November 12, 2024
Citation: Morales-León F, et al. (2024). Atorvastatin-Induced Liver Injury with Rapid Enzyme Elevation: A Case-Report. Mathews J Case Rep. 9(10):191.
Copyrights: Morales-León F, et al. (2024).
ABSTRACT
Background: Statins, particularly atorvastatin, are widely used for managing dyslipidemia and preventing cardiovascular events and stroke. Despite their well-documented efficacy, concerns over statin-induced liver injury (SILI), a subset of drug-induced liver injury (DILI), have appeared. Case: this details the clinical presentation, diagnosis, and management of atorvastatin-induced hepatotoxicity in a 55-year-old female patient following a non-ST elevation myocardial infarction (NSTEMI). After being treated with atorvastatin, our patient developed symptoms indicative of liver damage, confirmed by elevated liver enzymes and hepatocellular injury. Atorvastatin was discontinued, resulting in the normalization of liver function. Due to her high cardiovascular risk, rosuvastatin, an alternative statin with a lower hepatotoxicity profile, was introduced, and regular monitoring showed no further complications. Discussion: This case underscores the importance of balancing the cardiovascular benefits of statins against their potential hepatic risks. Clinicians must closely monitor liver function, especially in high-risk patients, and consider alternative statins when reinitiation is necessary. This report highlights the need for individualized treatment plans to mitigate statin-induced hepatotoxicity, ensuring both patient safety and cardiovascular protection.
Keywords: Drug Induced Liver Injury, Atorvastatin, Alanine Transaminase, Aspartate Aminotransferases, Alkaline Phosphatase, Case Report, Cardiovascular Disease, Biochemical Indices.
ABBREVIATIONS
ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferases; DAPT: Dual Antiplatelet Therapy; DILI: Drug Induced Liver Injury; GGT: Gamma-Glutamyl Transferase; MI: Myocardial Infarction; NSTEMI: Non-ST Elevation Myocardial Infarction; SILI: Statin-Induced Liver Injury.
GRAPHICAL ABSTRACT
INTRODUCTION
Statins are lipid-lowering agents with a strong body of evidence proving their benefits, including a decrease in all-cause mortality and reduction in cardiovascular events and stroke [1]. Atorvastatin, a widely prescribed statin, plays a crucial role in the management of dyslipidemia and the prevention of aforementioned events. Despite its well-established efficacy and widespread use, concerns regarding its hepatotoxic potential have emerged, with an increasing number of cases of drug-induced liver injury (DILI) being reported in the literature [2].
DILI refers to liver damage caused by prescription medications, over-the-counter drugs, or herbal supplements, and is a major concern for clinicians prescribing statins, particularly in high-risk patients [3]. Understanding the clinical implications of statin-induced liver injury (SILI), a DILI subset, is essential, particularly in post-stroke patients where statins are pivotal in secondary prevention [4].
Statins primarily work by inhibiting 3-hydroxy-methyl-glutaryl coenzyme A, which ultimately reduces cholesterol biosynthesis. However, the cardiovascular benefits of statins extend beyond their ability to lower lipid levels. These additional protective effects, known as "pleiotropic" effects, do not depend on reducing low-density lipoprotein cholesterol (LDL-C) alone. Numerous studies have consistently reported that statins stimulate nitric oxide expression, enhancing endothelial function. Moreover, they have significant anti-inflammatory properties reducing T-cell activation, decreasing macrophage infiltration, lowering vascular wall inflammation, and help stabilizing plaque. Statins also suppress several pro-inflammatory cytokines involved in atherosclerosis [5].
According to Ward et al. [6] the mechanisms of statin-induced hepatocellular injury are unclear, although animal studies suggest that the reduction in mevalonate or one of its sterol intermediates may be associated with an elevation in liver enzymes. Also, asymptomatic rises without histopathological changes may result from alterations in hepatocyte membrane lipid composition, leading to increased permeability and leaking of the liver enzymes. Statin-induced hepatotoxicity may also arise from extensive hepatic metabolism and lipophilicity, with a high oral daily dose associated with an increased risk of drug-induced liver injury.
Reports show that up to 3% of patients may develop a mild transaminase elevation within the first year of statin therapy but are rarely associated with symptoms and often resolve spontaneously [7].
A comparative study of 49 complete trials involving atorvastatin revealed that persistent elevation in hepatic transaminases more than 3 times the upper limit of normal (ULN) was rare, observed in 0.1%, 0.6% and 0.2% of patients treated with atorvastatin 10 mg, 80mg, and placebo, respectively [8]. The results show that liver toxicity appears to be a class effect, with the increased risk of elevated liver enzymes, with an increasing statin dose [9].
In Chile, the national Pharmacovigilance System has few reports related to gastrointestinal disturbances associated with atorvastatin, but no cases of altered hepatic enzyme levels.
This case report explores the clinical presentation, diagnosis, and management of atorvastatin-induced liver disease, with an emphasis on the ongoing monitoring required in patients with high cardiovascular risk. Additionally, it addresses the considerations surrounding statin reinitiation, including the potential use of alternative statins and the risk of cross-reactivity within this drug class.
CASE
A 55-year-old female patient with a history of dyslipidemia without pharmacology treatment, presented an episode of non-ST elevation myocardial infarction (NSTEMI). She was treated in the emergency department following standard MI protocols, including dual antiplatelet therapy (DAPT) with loading doses of clopidogrel and aspirin, atorvastatin 80 mg every day, and enoxaparin 40 mg every 12 hours. Two days later, the patient was transferred to the hemodynamics unit, where a drug-eluting stent was placed, leading to symptomatic improvement and clinical stabilization. At the kidney level, his tests did not indicate any alterations, with the blood urea nitrogen, serum creatinine and glomerular filtration rate at normal levels.
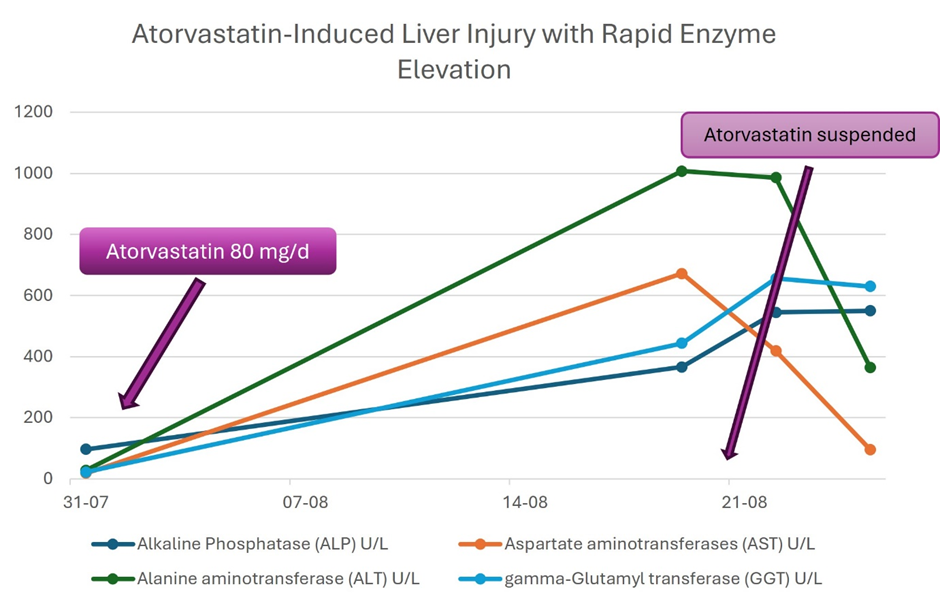
Following this, the patient was discharged with instructions to continue DAPT (aspirin 100 mg and clopidogrel 75 mg daily) and atorvastatin 80 mg once daily. Ten days post-discharge, she developed generalized malaise, nausea, anorexia, and abdominal pain, prompting a return to the emergency department. Initial laboratory tests were performed, after which she was discharged with recommendations for rest and analgesics. Four days later, the patient's symptoms worsened, resulting in a second visit to the emergency department. Further investigations, including an abdominal ultrasound, soft tissue evaluation, and laboratory tests (complete blood count and liver function profile), revealed elevated liver enzymes, with hepatocellular damage (R:7,3) with negative viral hepatitis serologies, HCV RNA and autoimmune hepatitis serologies (Table 1). The imaging studies were suggestive of drug-induced liver injury (DILI), due to atorvastatin.
In response to these findings, atorvastatin was discontinued [10]. Roussel Uclaf Causality Assessment Method (RUCAM) was applied to assess causality, classifying the adverse drug reaction as “probable” and the score was 7 [11]. After discontinuing atorvastatin for 15 days, serial liver function tests demonstrated stabilization and subsequent normalization of liver enzyme levels.
Once acute hepatotoxicity was resolved, considering the patient’s high cardiovascular risk (as calculated), it was decided to reintroduce statin therapy with rosuvastatin 40 mg daily. Serial liver function tests were conducted in 3 and 6 months, confirming the normalization of hepatic enzymes without further complications.
Table 1. Laboratory results tracking from initial non-ST-elevation myocardial infarction (NSTEMI) diagnosis to final medical evaluation (Year 2022)
|
Exams by Date |
Normal Values |
NSTEMI |
Second Visit to an Emergency Unit |
First Control |
Second Control |
|---|---|---|---|---|---|
|
|
31/07 |
19/08 |
22/08 |
25/08 |
|
|
Total Bilirubin (mg/dL) |
0.0 – 1.0 |
0.25 |
0.40 |
0.40 |
|
|
Direct Bilirubin (mg/dL) |
≤ 0.3 |
< 0,10 |
0.30 |
0.30 |
|
|
Alkaline Phosphatase (ALP) U/L |
44 – 140 |
97 |
366 |
545 |
550 |
|
Aspartate aminotransferases (AST) U/L |
8 – 33 |
19 |
672 |
419 |
96 |
|
Alanine aminotransferase (ALT) U/L |
4 – 36 |
28 |
1007 |
986 |
365 |
|
Total proteins (g/dL) |
6 – 8.3 |
6.7 |
6.6 |
|
|
|
Albumin (g/dL) |
3.4 – 5.4 |
3.72 |
3.9 |
3.9 |
4.0 |
|
gamma-Glutamyl transferase (GGT) U/L |
5 – 40 |
22 |
444 |
656 |
630 |
|
Prothrombin Time (seconds) |
10 – 15 |
12.2 |
|
|
10.1 |
|
Uremia (mg/dL) |
6 – 20 |
42.8 |
|
34 |
38 |
|
Calcium (mg/dL) |
8.5 – 10.2 |
8.3 |
|
8.7 |
|
|
Glucose (mg/dL) |
70 – 99 |
153 |
|
|
|
|
Lipase (U/L) |
13 – 60 |
35 |
|
|
|
|
Lactate Dehydrogenase (LHD) (U/L) |
135 - 214 |
202 |
|
427 |
217 |
|
Reactive C Protein (mg/dL) |
< 0.5 |
0.72 |
6.9 |
|
17.8 |
|
Nuclear Antigens |
|||||
|
Ap100 (AC-6) |
|
|
|
|
positive +++ |
|
PML (AC-6) |
|
|
|
|
positive +++ |
|
IgA (mg/dL) |
40 – 350 |
|
|
|
159 |
|
IgG (mg/dL) |
650 – 1600 |
|
|
|
1260 |
|
IgM (mg/dL) |
54 – 300 |
|
|
|
197 |
|
Anti liver autoimmune antigen antibody |
|||||
|
Anti mitochondrial (M2) ac-21 |
|
|
|
|
positive + |
|
Sp100 (AC-6) |
|
|
|
|
positive +++ |
|
Anti PML (AC-6) |
|
|
|
|
positive ++ |
|
Anti-nuclear cytoplasmatic antibody |
|||||
|
Ac. antinuclear |
|
|
|
|
positive |
|
ANA title |
|
|
|
|
1/1280 |
DISCUSSION
Atorvastatin-induced liver injury can manifest in a variety of clinical presentations, ranging from asymptomatic elevations in liver enzymes to more severe outcomes, including acute hepatic failure. Management typically involves the immediate discontinuation of the offending agent and close monitoring of liver function. However, in the context of secondary prevention, the decision to reinitiate statin therapy generates a significant challenge. Clinicians must carefully weigh the benefits of preventing further cardiovascular events against the risk of potential liver injury recurrence [12].
The US Drug-Induced Liver Injury Network (DILIN) is a multicenter prospective study group aiming to evaluate DILI in the United States from 2004 onward. They report that SILI is rare, mild to moderate in intensity, self-limited, and represents a class effect with variable presentation from hepatocellular pattern, to cholestatic, and even autoimmune hepatitis-like phenotypic manifestations [13].
The diagnosis of atorvastatin-induced liver disease is often based on the temporal association between the initiation of statin therapy and the onset of liver dysfunction, while ruling out other potential etiologies. This case report highlights the importance of maintaining a high index of suspicion, especially in post-stroke patients who may present additional risk factors for liver injury, such as polypharmacy or pre-existing liver conditions [14].
The temporal relationship between statin initiation and the onset of liver injury, coupled with the exclusion of alternative causes and the application of causality assessment tools RUCAM and his electronic version as RECAM [7], facilitated the diagnosis in this case.
Reports show that latency from the time of initial statin exposure to clinical evident DILI varied widely, from 34 days to more than 10 years. Statin associated hepatocellular injury frequently occurs 5 to 90 days after initiation of therapy, with predominant rise in aminotransferases, specifically alanine aminotransferase (ALT), while cholestatic pattern is associated with a rise in alkaline phosphatase (ALP) and bilirubin. Bilirubin levels of more than twice the ULN imply severe hepatocellular liver injury with a mortality of 10% [15]. This case shows a mixed cholestatic hepatocellular pattern with elevation of ALT and ALP without elevation of bilirubin levels.
The adverse event management involved immediate discontinuation of atorvastatin, leading to the resolution of hepatotoxicity. However, the challenge lies in balancing the benefits of statin therapy for cardiovascular risk reduction against the risks of recurrent liver injury. In cases where reinitiation of statin therapy is considered necessary, alternative statins such as rosuvastatin, which has a lower propensity for hepatotoxicity, may be an appropriate choice.
Close monitoring of liver function is crucial to ensure the safety and efficacy of the reintroduced therapy, given that rosuvastatin has a lower risk of DILI, even though there are reports associated with this effect [10].
This case reminds us of the importance of regular hepatic monitoring and individualized treatment plans to mitigate the risks associated with statin-induced liver injury, especially in patients at high cardiovascular risk.
CONCLUSION
This case report highlights the rare but significant risk of atorvastatin-induced liver injury, a form of DILI, in a post-stroke patient undergoing secondary prevention for cardiovascular events. Statins are essential in reducing cardiovascular morbidity and mortality, but clinicians must remain vigilant regarding potential hepatotoxic effects, especially when prescribing high-dose statins in patients with other risk factors of liver dysfunction.
AUTHOR CONTRIBUTION
Conceptualization: FML
Data collection: FML, CS
Case Analysis: FML, CS, PFR, TS, EV
Writing and original draft preparation: FM
Writing review and editing: TS, PFR, EV
Supervision: EV
ACKNOWLEDGEMENTS
None.
CONSENT FROM PATIENT
Written as well as verbal consent taken.
CONFLICT OF INTEREST DECLARATIONS
None.
REFERENCES
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, et al. (2022). Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 328(8):746-753.
- Saha A, Garg A. (2021). Severe Liver Injury Associated with High-Dose Atorvastatin Therapy. J Investig Med High Impact Case Rep. 9:23247096211014050.
- Li M, Luo Q, Tao Y, Sun X, Liu C. (2022). Pharmacotherapies for Drug-Induced Liver Injury: A Current Literature Review. Front Pharmacol. 12:806249.
- Thalmann I, Preiss D, Schlackow I, Gray A, Mihaylova B. (2023). Population-wide cohort study of statin use for the secondary cardiovascular disease prevention in Scotland in 2009-2017. Heart. 109(5):388-395.
- Kadoglou NPE, Stasinopoulou M. (2023). How to Use Statins in Secondary Prevention of Atherosclerotic Diseases: from the Beneficial Early Initiation to the Potentially Unfavorable Discontinuation. Cardiovasc Drugs Ther. 37(2):353-362.
- Ward NC, Watts GF, Eckel RH. (2019). Statin Toxicity. Circ Res. 124(2):328-350.
- Meurer L, Cohen SM. (2020). Drug-Induced Liver Injury from Statins. Clin Liver Dis. 24(1):107-119.
- Newman C, Tsai J, Szarek M, Luo D, Gibson E. (2006). Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol. 97(1):61-67.
- Onfiani G, Nascimbeni F, Carubbi F. (2021). A case of statin-induced liver injury with positive rechallenge with a second statin. Is there a class effect? J Basic Clin Physiol Pharmacol. 32(6):1151-1155.
- Björnsson ES. (2021). Clinical management of patients with drug-induced liver injury (DILI). United European Gastroenterol J. 9(7):781-786.
- Hayashi PH, Lucena MI, Fontana RJ, Bjornsson ES, Aithal GP, Barnhart H, et al. (2022). A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology. 76(1):18-31.
- Kashyap K, Bisht K, Dhar M, Mittal K. (2023). Atorvastatin-induced Myositis and Drug-induced Liver Injury. J Assoc Physicians India. 71(10):96-98.
- Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. (2014). Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 60(2):679-686.
- Khan AA, Ahmed S, Mohammed A, Elzouki AY. (2020). Autoimmune-like Drug-induced Liver Injury Caused by Atorvastatin and Demonstration of the Safety Profile of Pravastatin: A Case Report and Literature Review. Cureus. 12(3):e7299.
- Averbukh LD, Turshudzhyan A, Wu DC, Wu GY. (2022). Statin-induced Liver Injury Patterns: A Clinical Review. J Clin Transl Hepatol. 10(3):543-552.