Current Issue Volume 8, Issue 1 - 2024
Assessment of the Toxicity and Histopathological Effects of Launaea taraxocifolia Extract on Fingerlings of Clarias gariepinus
George UU1,*, Ajayi OO2, Essien-Ibok MA3, Abiaobo NO4, Mbong EO5
1Department of Fisheries and Aquaculture, Faculty of Agriculture, Akwa Ibom State University, Obio Akpa Campus, Akwa Ibom State, Nigeria
2Department of Pathology, Faculty of Basic Clinical Sciences, University of Uyo, Akwa Ibom State, Nigeria
3Department of Fisheries and Aquatic Environmental Management, University of Uyo, Nigeria
4Department of Zoology, Akwa Ibom State University, Ikot Akpaden, Nigeria
5Department of Environmental Biology, School of Science and Technology, Heritage Polytechnic-Eket, Akwa Ibom State, Nigeria
*Corresponding Author: George UU, Department of Fisheries and Aquaculture, Faculty of Agriculture, Akwa Ibom State University, Obio Akpa Campus, Akwa Ibom State, Nigeria; Email: [email protected]
Received Date: April 12, 2024
Publication Date: April 24, 2024
Citation: George UU, et al. (2024). Assessment of the Toxicity and Histopathological Effects of Launaea taraxocifolia Extract on Fingerlings of Clarias gariepinus. Mathews J Cytol Histol. 8(1):27.
Copyright: George UU, et al. © (2024)
ABSTRACT
The toxicity of ethanolic extract of Launaea taraxocifolia leaves was evaluated in Clarias gariepinus fingerlings over a 96-h exposure. The fish were exposed to 22 mgL-1, 23 mgL-1, 24mgL-1, and 25 mgL-1 of the extract and a control in an acute static toxicity bioassay after performing a range finding test to determine the median lethal concentration (LC50) of the extract. Exposed fish showed signs of behavioural abnormalities, histopathological alterations including mortality. The appearance and intensities of the observed signs were concentration and exposure period-dependent. An LC50 value of 22.4 mg/l representing a log transformed concentration of 1.35 mg/l was established for the extract in the experimental groups. The low 96-hr LC50 values of L. taraxocifolia recorded for C. gariepinus fingerlings suggest that it is highly toxic and hence under field application the toxicant can have adverse effects on non-target species. However, it can be efficiently applied in ponds to eradicate predators, competitors and unwanted fish populations.
Keywords: Launaea taraxocifolia, Clarias gariepinus, Toxicity, Histopathology, Fingerlings
INTRODUCTION
Launaea taraxocifolia, commonly known as wild lettuce is a versatile and resilient plant that has captivated human attention for centuries. Launaea taraxocifolia a member of the Asteraceae family, distinguished by its characteristic’s yellow flowers and deeply toothed leaves. It’s taproot system allows it to thrive in a variety of soil conditions, contributing to its widespread distribution across the globe. Despite being native to Europe and Asia, wild lettuce has successfully established in various ecosystems, from meadows to urban environments (Adebisi, 2000) [1].
Historically, L. taraxocifolia have been utilized for their medicinal properties. Traditional herbal medicine recognizes the plants for its diuretic, anti-inflammatory and anti-oxidant qualities (Owoeye, et. al., 2015) [2]. The leaves, roots and flowers of L. taraxocifolia have been employed to treat ailments ranging from digestive issues to liver problems (Schippmann, et. al., 2006; Uiyal, et. al., 2006) [3,4].
Beyond human uses, L. taraxocifolia plays a crucial ecological role. Its flowers provide nectar for pollinators, supporting biodiversity. They deep taproots help break up compacted soil, promoting better water absorption and soil health. Additionally, the plants’ ability to colonize disturbed areas contributes to ecological succession.
Studying the toxicological effects of L. taraxocifolia (wild lettuce) on fingerlings of Clarias gariepinus (African catfish) is important for several reasons. Firstly, it helps assess potential risks to aquatic ecosystem if wild lettuce extracts or components are introduced. Understanding toxicity level is crucial for environmental conservation. Secondly, fingerlings of Clarias gariepinus are often used in aquaculture, toxicological studies provide insights into any harmful effects that exposure to L. taraxocifolia might have on the growth, development or overall health of this fish, which is vital for sustainable aquaculture practices. Lastly, these studies will contribute to broader knowledge about these interactions between L. taraxocifolia and organisms, aiding in the development of guidelines for responsible use of herbal extracts or plant-based components in aquatic environment.
Artisanal fishermen use plant extract as part of their artisanal fishing tools (Power et al., 2010) [5]. Extracts of plants such as Blighia sapda, Kigelia africana, Raphia, vinifera (Omoitoyin et al., 1999) [6], Derris elliptica, Tephrosia vogelli (Oluwatoyin, 2011) [7] and Balanites aegyptiaca (Wakawa et al., 2018) [8] have been reported to be used by fishermen as fishing tool. These plant extracts used in harvesting fish have toxic properties (Fafioye et al., 2004) [9] that paralyze or stupefy fish (Fafioye, 2011) [10] in the aquatic environment. Examination of the phytochemicals of plants used as fish poison shows the presence of saponins, alkaloid and flavonoids (Fafioye, 2011) [10]. Others are tannins, resins, terpens, cardiac glycosides and balsam (Wakawa et al., 2018) [8]. Saponins affect haematology and oxygen uptake of fish (Roy and Munshin, 1989) [11] while alkaloid and flavonoids have anaesthetic properties on fish (Tsuchiya, 2017) [12].
Plants extracts can have various effects on water quality, fishes, zooplankton community and macroinvertebrate abundance depending on the specific properties of the extract and the environmental conditions. Plants extracts can release organic compounds into the water, affecting nutrients levels and dissolved oxygen content, pH levels and turbidity, thereby altering water clarity (Jonah and George, 2019; Jonah, et. al., 2019; Jonah, et. al., 2020) [13-15].
Stem bark of A. leiocarpus contains phytochemicals such as tannins, flavonoids, terpens and saponins with absence of alkanoids and anthraquinones (Salau et al., 2013) [16]. Introduction of plant extracts containing these phytochemicals could result into physiological stress in aquatic biota which could ultimately reduce aquatic productivity (Oluwatoyin, 2011) [5] or even death. There is paucity of information on the effects of ethanolic extracts of common wild lettuce (L. taraxocifolia) on the survival and histopathology of gills of Clarias gariepinus fingerling. Thus, this study seeks to investigate the toxicity of this plants. Assessing these impacts helps in understanding and managing ecological balance. Regulatory agencies often require toxicological assessment of potential pollutants or contaminants. Understanding the toxicological impacts of Launaea taraxocifolia on C. gariepinus fingerlings aids in regulatory compliance and decision-making regarding the use or control of T. officinale in aquatic environments.
MATERIALS AND METHODS
Collection of Test Organism
Fingerling of Clarias gariepinus were collected from Akwa Ibom State University fish farm, Obio Akpa Akwa Ibom State, Nigeria located within latitude 5°17′N and 7°27′N, Longitude 7°27′E and 7°58′E. The climate of the area is tropical and is characterized by distinct wet and dry seasons (George et.al., 2023a, b) [17,18]. The vegetation of the study area is generally rainforest close to the mangrove belt. Human activities in the area include farming, hunting, boat building and sand mining. A total of two hundred (200) fingerling were collected and used for the study.
Acclimatization of Specimen's
The fingerlings were acclimated in a re-circulatory plastic aquarium measuring 25 ×13 × 8.3 Cm3 containing hatchery water for 24hours in the fisheries and aquaculture laboratory of Akwa Ibom State fish farm. This enhanced the stability of the fingerlings from stress of collection and transportation (Udo et al, 2006) [19].
Collection of Plant Sample
Fresh leaves of wild lettuce (Launaea taraxocifolia) were collected for the study. The collection site of the plant was Atan offot in Uyo Local Government Area, Akwa Ibom State. The date of Collection was 20th November, 2023. The plants material was transported to University of Uyo, Uyo, Akwa Ibom State for identification and authentication of the plants. This was done at the Herbarium in the Department of Botany, University of Uyo, Uyo with herbarium No: (udobot, UUH 4419 (Uyo).
Preparation of Plant Material
After the identification, the leaves were washed and sun dried. The leaves were shredded and spread on cellophane and allowed to dry for 72 hours under room temperature. The dried leaves were pulverized (grinded) into fine powder using wooden pestle and mortar.
Preparation of Ethanolic Extract (Maceration and Extraction)
Cold extraction method (Maceration) was used in this research according to Hidayat and Wulandari (2021) [20], in the extraction procedure, 1000ml of 99% Concentrated Ethanol was used to Macerate 240g of the plant materials in an airtight container and kept in the laboratory under room temperature for 72 hours (3 days). The ethanolic suspension was filtered using a filter net and filter paper and the extract was evaporated in a water bath at 40° Celsius for 48 hours and stored in a beaker covered with aluminum foil for bioassay immediately after the evaporation was complete.
Preparation of Experimental Aquaria
Ten (10) rectangular plastic aquaria measuring 25 ×10 × 15 cm were thoroughly washed with tap water and properly rinsed with fresh water of similar salinity and allowed to drain dry for 24 hours on the laboratory bench based on Dede and Kagbo (2001) [21].
Stocking of Specimen
Prior to commencement of actual experiment, a preliminary test or range finding test with varying concentration (0, 5, 10, 15, 20) was conducted to give the actual variations in concentration to be used for the bioassay. Each of the aquarium had a replicate to ensure accuracy. Each of the Ten (10) plastic aquaria was filled with two liters of hatchery water and 10 Clarias gariepinus fingerlings was stocked in each aquarium. The ethanolic extract of wild lettuce (L. taraxocifolia) with varying concentrations (0, 22, 23, 24, 25) was added to each stocked aquaria and allowed to stand for 96 hours for mortality examination.
Monitoring of Water Quality
Water Quality Parameters was monitored prior to commencement of the experiment and also periodically according to Standard Method (APHA, 1998) [22]. Parameters that were monitored include dissolve Oxygen (DO), pH, And Temperature (0C). Temperature and pH were measured using portable pH /Ec/ TDs/ Temperature HANNA, H1 991301 Model instrument while oxygen was measured using digital portable analyser JPB - 607A from "Search Tech Instrument".
Monitoring of Specimen for Mortality
The effects of the various concentration of the ethanolic extract of wild lettuce (L. taraxocifolia) on the fingerlings was monitored on a 24 hours’ basis for 96 hours as recommended by Udo et. al., (2006) [23] and Ekanem and Ekpo (2008) [24].
Determination of Mortality and Survival Rates of Fingerlings
The percentage mortality and survival rates of the fingerlings in the different concentrations of the ethanolic extract of L. taraxocifolia during the period of study was determine using the formula
% mortality =n/N × 100 (Chan, 1977) [25].
Where;
n = number of dead fish per aquarium per concentration
N = Total Individual Stocked
The difference between dead fish and survivors will give the percentage survival of the fingerlings at the end of the experiment (96 hours) (Udo et. al., 2006) [20].
Determination of Mortality Lethal Median Concentration (96 Hours Lc50)
The effects of the various concentrations of the ethanolic extract of plant (L. taraxocifolia) on the fingerlings of C. gariepinus was determined by graphical method (Probit Level Determination as recommended by Omoregie (2002) [26], Omoregie and Ufodike (2000) [27], Ekanem and Ekpo (2008) [24] and Udo et.al. (2006) [20]. At Lethal Median Concentration LC50, after 96 hours of test, the number of fingerlings that are expected to die was determined from the graph. Similarly, the concentration that will kill 50% of the stocked fingerlings at the end of the test (96 hours) was determined at the probit level (Omoregie, (2002) Omoregie and Ufodike (2000), Udo et. al., (2006) [20]; Ekanem and Ekpo (2008) [24].
Collection of Samples for Histopathological Examinations
The gill's tissues were isolated from the test animal and fixed in formalin -saline for 48 hours. The fixed tissue was processed manually through graded ethanol, cleared in xylene impregnated and embedded in paraffin wax, sections of the tissue sample were cut with a rotary microtome, stained by hematoxylin and eosin technique, prepared tissues were finally observed using a microscope for pathological changes at x100 and x400 magnification.
Data Analysis
The results of the respective concentration effects of the ethanolic extract of L. taraxocifolia was presented in tables. One-way analysis of variance (ANOVA) was used to test for significant difference between the varying concentrations in both batches (batch A and batch B) at the probability level of (P>0.05). Probit analysis was done using SPSS version 20.0.
RESULTS
Initial Water Quality Parameters
Prior to commencement of the experiment, basic water quality parameters were measured and the values were dissolved oxygen (5.8 mg/l), temperature (29.50C) and pH (6.40). All the parameters measured were observed to fall within the acceptable range for aquaculture operations.
Table 1: Initial Physico-chemical parameters of the test water prior to stocking of test organism.
|
Fish Species |
Initial physico-chemical parameters prior to stocking |
||
|
DO (mg/l) |
Temp (oC) |
pH |
|
|
Clarias gariepinus |
5.8 |
29.5 |
6.40 |
Variation of in Physico chemical Parameters of Test Media
The effect of L. taraxaxifolia leaf extract on the physico-chemical properties of the culture medium was assessed in the study (Table 2). Based on the findings of this study, values for temperature and hydrogen ion concentration (pH) recorded revealed no substantial different (p > 0.05) among treatments with reference to the control, but considerable changes (p < 0.05) were observed for dissolved oxygen.
Table 2: Mean Physico-chemical properties of the test media across treatments.
|
Conc. (mg/l) |
Parameters |
||
|
Dissolved Oxygen (mg/l) |
Temperature (0C) |
pH |
|
|
0 (control) |
4.95±0.75a |
27.93±0.9 a |
6.27±0.1a |
|
21 |
3.88±2.4b |
27.50±1.2 a |
6.25±0.06a |
|
22 |
3.60±2.00b |
27.18±0.6 a |
6.22±0.08a |
|
23 |
3.25±2.00b |
27.08±0.7 a |
6.20±0.09a |
|
24 |
3.18±2.00b |
27.03±0.8 a |
6.15±0.05a |
Mean with different superscripts along the same column are significantly different at p
Summary of the Percentage Mortality and survivors of C. gariepinus Fingerlings in the different concentrations of the ethanoic extract of Launaea taraxocifolia at the end of the experiment (96 hrs.)
The percentage mortality and survivors of C. gariepinus fingerlings at the end of the test period in each of the concentrations are shown in Table 3 for the two batches of the experiment.
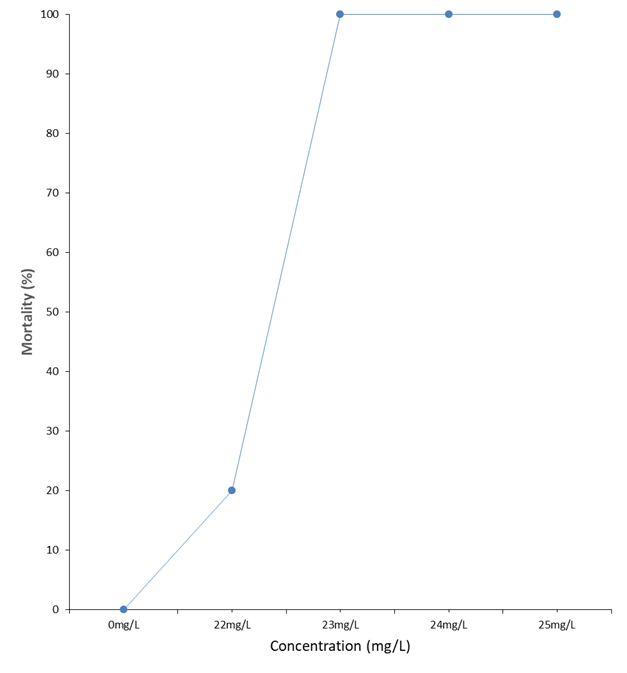
In the 0 mg/l concentration of the extract, no mortality was recorded throughout the test period in both batches A and B. in the 22 mg/l concentration of the extract, 20 % mortality was recorded leaving behind 80 % survivors in both bathes.
At the end of the 96-hour bioassay 100 % mortality was observed in the 23, 24 and 25 mg/l concentration of the extract leaving behind no test organisms in the test media for both batches (Table 3). Statistical Analysis using one-way Anova (SPSS 20.0) showed that there was no significant difference (p>0.05) in mortality between the two batches.
Table 3: Summary of the Percentage Mortality and survivors of C. gariepinus Fingerlings in the different concentrations of the ethanoic extract of Launaea taraxocifolia at the end of the experiment (96 hrs.).
|
Conc. of extract (mg/l) |
BATCH A |
BATCH B |
||||||
|
Mortality (M) |
% M |
Survivors (S) |
% S |
Mortality (M) |
% M |
Survivors (S) |
% S |
|
|
0 |
0 |
0 |
10 |
100 |
0 |
0 |
10 |
100 |
|
21 |
2 |
20 |
8 |
80 |
2 |
20 |
8 |
80 |
|
22 |
10 |
100 |
0 |
0 |
10 |
100 |
0 |
0 |
|
23 |
10 |
100 |
0 |
0 |
10 |
100 |
0 |
0 |
|
24 |
10 |
100 |
0 |
0 |
10 |
100 |
0 |
0 |
96 Hours LC50 Determination
The 96 hours LC50 for C. gariepinus fingerlings exposed to the different concentrations of the ethanolic extract of Launaea taraxocifolia was determine using probit analysis. The concentrations were first transformed into log for the probit analysis (Table 4). The 96 hours LC50 is given at 22.4 mg/l representing a log transformed concentration of 1.35 mg/l a point where 50 % of the test organisms are expected to die at the end of the 96th hours bioassay (Fig 1).
Table 4: LC50 determination for C. gariepinus Fingerlings at the end of the 96-hours bioassay.
|
Concentration (mg/l) |
Log Transformation |
Mortality (M) |
% Mortality |
Survivor (S) |
% Survivor |
|
0 |
0 |
0 |
0 |
10 |
100 |
|
22 |
1.34 |
2 |
20 |
8 |
80 |
|
23 |
1.36 |
10 |
100 |
0 |
0 |
|
24 |
1.38 |
10 |
100 |
0 |
0 |
|
25 |
1.40 |
10 |
100 |
0 |
0 |
Histopathology of the gill of C. gariepinus Exposed to the different concentrations of the Ethanolic extract of Launaea taraxocifolia
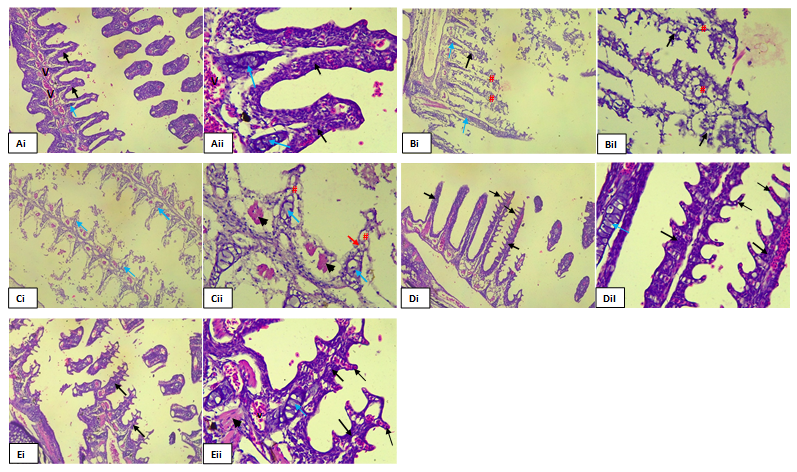
The histological analysis was on the gills of Clarias gariepinus exposed to varying concentrations of the Ethanolic extract of Launaea taraxocifolia to evaluate potential histological alterations was shown in figure 2. The results of the histological analysis revealed distinct histological changes among the experimental groups exposed to different concentrations of the Ethanolic extract, as compared to the control group.
Gills from the control group exhibited normal histological features, characterized by intact epithelial architecture with no apparent pathological changes. Specimens exposed to lower concentrations of the Ethanolic extract (21mg/L and 22mg/L) displayed diffuse epithelial degeneration. Histological examination revealed widespread vacuolation and severe loss of epithelial cells, indicating significant damage to the gill epithelium compared to the control group.
In contrast, specimens exposed to higher concentrations of the Ethanolic extract (23mg/L and 24mg/L) exhibited normal histological architecture comparable to the control group. No significant histological changes were observed, suggesting minimal impact on gill tissue integrity at these concentrations.
Figure 2. Photomicrograph of gills tissue section of the control group A (i&ii) showing highly cellular Primary lamella and secondary filament at 0 mg/l of the extract, B(i&ii) gills tissue section showing highly cellular Primary lamella (thick arrow) and secondary filament (thin arrow) at 21 mg/l of the extract, C (i&ii) and D (i&ii) showed diffused degeneration of the gills epithelium (#) consisting predominantly the gill cartilage epithelial tissue at 22 mg/l, 23 mg/l and 24mg/l concentration of the extract respectively.
DISCUSSION, CONCLUSION, RECOMMENDATIONS
Discussion
Physico-chemical parameters of the Experimental Water
Three basic physico-chemical parameters were taken in line with standard practice in toxicological studies prior stocking of the experimental fish. Dissolved Oxygen value of 5.8 mg/l with a value of 29.5oC recorded for temperature and a value of 6.97 was recorded for pH.
The water quality parameters were found to be within the recommended standards for aquaculture (Udo, 2007, Ajah 2007, George et.al., 2013a; George et.al., 2013b; George et.al., 2014a; George et.al., 2015a; George, et. al., 2015 c) [23, 28-33] prior to conducting the toxicity test. This suggests that the water was of sufficient quality to support aquatic life and that there were no significant levels of pollutants present that could harm the organisms being tested. This is crucial because conducting toxicity test in water with poor water quality could yield inaccurate results or cause harm to the test organisms.
During the experimental period variations in the physico-chemical parameters were generally observed in the experimental aquaria in both batches. The values of physico-chemical parameters varied depending on time and concentration. As the concentration of the toxicant increased with time, the values of the physico-chemical parameters were observed to fluctuate when compared with the control. Variations in water quality during the 96-hours bioassay involving Lunaea taraxifolia and fingerlings of Clarias gariepinus could be due to several factors. When a toxic substance like L. taraxifolia is introduce into the aquatic environment or a test media, it can alter the water quality parameters such as pH, dissolved oxygen levels and temperature. These changes can directly affect the survival, growth and behaviour of the fingerlings.
Moreover, the presence of pollutants can alter the pH of the water, making it more acidic or alkaline, which can stress the fish and affect their physiological processes. Temperature fluctuations can also impact fish metabolism and oxygen solubility in water, further affecting their health. Variations in water quality during bioassay is likely to occurred as a result of the interaction between the toxicant, test organisms and the surrounding environment, highlighting the complex dynamics involved in toxicity studies.
This phenomenon has been previously reported in the physico-chemical parameters of the test water media by George, et. al., (2013a) [29] when reporting on the effects of lethal concentration of rubber extract (Hevea brasiliensis) on the survival of fingerlings of Clarias gariepinus under laboratory conditions. George, et. al., (2013b) [30] during their studies on laboratory bioassay of the potential effects of rubber extract (Hevea brasiliensis) on the survival of fingerlings of Oreochromis niloticus, George, et. al., (2014a) [31] when reporting on the acute toxic effect of qua Iboe light crude oil on the gills of Clarias gariepinus Juveniles, and George, et.al., (2015a) [32] when evaluating the toxic effect of crude oil on hatchery reared Oreochromis niloticus fingerlings.
Preliminary Test of Ethanolic Extract of Lunaea taraxifolia on Clarias gariepinus Fingerlings
The importance of preliminary findings in toxicity studies is to help determine the appropriate dosage levels of Lunaea taraxifolia to be used in the main toxicity test (George, et.al., 2013a, 2013b; George, et. al., 2014b; George, et. al., 2015 a; 2015 b) [30-34]. By exposing the organisms to varying concentrations of the substance, a dose response relationship can be established and save concentrations that elicit measurable effects without causing excessive harm to the test organisms will be selected George, et.al., 2013a, 2013b; George, et. al., 2014b; George, et. al., 2015 a; 2015 b) [30-34]. Also, conducting a preliminary test validates the experimental methods and procedures to be used in the main toxicity test. It ensures that the experimental set-up, exposure duration, sampling handling and data collection protocols are accurate, reliable and reproducible.
Though toxicity range values are usually found to be different for each toxicant and organisms (Bossayt and Jansen, 2005) [35], the procedure is generally acceptable in Eco-toxicity experiments (APHA, 1998) [22].
No mortality was recorded in the 0 mg/l (control) concentration of the toxicant used for the bioassay. Similarly, no mortality was recorded in the experimental groups (5, 10, 15 and 20 mg/l) concentration of the extract leaving behind 100% survivors. This gave the range of doses to be used for the toxicity test.
Percentage Mortality and Survivors
Toxicity studies involving Lunaea taraxifolia on fingerlings of C. gariepinus showed an interesting result with mortality ranged of 0 – 100 % in both batches A and B at the end of the 96-hours bioassay. In the control (0 mg/l) group no mortality was recorded leaving behind 100% survivors. However, 20 % mortality was recorded in the 22 mg/l concentration of the extract in each of the batches leaving behind 80% survivors while 100 % mortality was recorded in each of the 23, 24 and 25 mg/l concentration of the extract. The results of the present findings are in consonance with the earlier reports by George et.al., (2023 a) [17] during their studies on establishing a dose-response toxicity for Clarias gariepinus fingerlings exposed to ethanolic extract of Latana camara; George et.al., (2023 b) [18] during their investigation on In Vivo Studies on mortality and histopathological indices of Phragmenthera capitata (Mistletoes) on Clarias gariepinus fingerlings in aquarium; George et.al., (2023 c) [36] when working on studies on mortality and histopathological alteration on the Gills of Oreochromis niloticus juveniles following exposure to ethanolic extract of Phramenthera capitata under laboratory conditions and earlier assertion made by George et.al., (2023 d) [37] when investigating on the dose-response relationship and histo-morphological alterations on Oreochromis niloticus juveniles following exposure to ethanolic extract of Latana camara.
In the present study, percentage mortality was concentration dependent. The higher the concentration, the higher the percentage mortalities. Similar results have been reported by different authors; Ogundiran et.al., (2010) [38] when investigating toxicological impacts of detergents effluents in juveniles of African catfish (Clarias gariepinus), Calta, et.al., (2004) [39] when studying the acute toxicity of the synthetic pyrethroid deltamethrin to young minnow carp (cyprinus carpio), Ayuba et. al., (2002) [40] when investigating on the acute toxicity of the root of Jimson’s weed (Datura innoxia) to the African catfish (Clarias gariepinus) fingerlings and Adedeji et.al., (2008) [41] when investigating acute toxicity of diazinion to African catfish (Clarias gariepinus) fingerlings.
The results of the present studies revealed that toxic effects were more pronounce at the 24-hours mark of the test. This suggests that fingerlings of C. gariepinus may experience a shock when exposed to L. taraxifolia, leading to more pronounced toxic effects. This acute exposure can overwhelm the fish detoxification mechanisms and physiological processes, resulting in visible signs of toxicity. Also, the concentration of the toxicant present in water might be higher at the beginning of the test, contributing to increased toxicity levels. As time progresses, the concentration of the toxin could decrease due to dilution or degradation processes, leading to reduced toxicity effects. The results of this findings agree favourably with earlier assertions by (Calta, et.al., 2004; Adedeji et.al., (2008); Ogundiran et.al., 2010; Ayotunde et.al., 2011, Essien-Ibok, 2020) [38-43].
96 Hours LC50
The 96 hours LC50 is known to vary with respect to different toxicants and concentrations due to various factors such as mode of action of the toxicant, the sensitivity of the organisms being tested and the specific environmental conditions under which the test is conducted. Different toxicants may have different mechanisms of actions, affecting organisms in distinct ways. Therefore, the variability in LC50 values reflects the complexity of interactions between toxicants and organisms in different conditions (Laguan et.al., 2004; Ayotunde et.al., 2010, George et.al., 2013a; 2013b, 2014a and 2015a) [29-32,42-45].
In the present study the 96 hours LC50 was given at 22.4 mg/l representing a log transformed concentration of 1.35 mg/l a point where 50 % of the test organisms are expected to die at the end of the 96th hours bioassay. The 96 hours LC50 of toxicants are known to vary as previously reported by the authors under reference. For instance, George et. al., (2013 a) [29] reported 96 hours LC50 of 50.12 mg/l for batch A and B Clarias gariepinus fingerlings under the toxicity effects of Hevea brasiliensis; 96 hours LC50 of 28.50 mg/l was reported by George et.al., (2013 b) [30] when working on the potential effects of Hevea brasiliensis on Oreochromis niloticus fingerlings. Again, George et. al., (2014) [31] reported the 96 hours LC50 of 30.12 mg/l on Clarias gariepinus juveniles using crude oil as the toxicant. The varied 96 hours LC50 values usually obtained from different toxicants and test organisms is again reported by George et. al., (2015a) [32], when they reported a 96 hours LC50 of 20 mg/l for Oreochromis niloticus fingerlings exposed to toxic effects of crude oil.
The variation observed in the 96 hours LC50 value of 22.4 (1.35) mg/l obtained for both batch A and B from previous studies affirmed earlier assertion by the authors cited above, that LC50 values depends on the ranges of concentration use for the toxicity test, test organism, the toxicant used and the environmental conditions under which the test is been conducted.
Pathological Effects of the Extract on the gills of the test Organisms
The effects of the ethanolic extract of L. taxaxifolia showed pathological effects on the gill lamellae of Clarias gariepinus fingerlings. However, the gill lamellae in the control (0 mg/l) group did not show any pathological changes. Pathological effects were pronounced at lower concentrations of 22 and 23 mg/l of the extract showing evidence of diffused epithelial degeneration of the primary and secondary gill lamella. There were no pathological variations in gills of the test organism at 24 and 25 mg/l of the extract.
However, the results of the present studies deviate from previous studies which shows that pathological changes were concentration-dependent. The histological changes observed in the present study were not dependent on the concentration as severe alteration were pronounced at lower concentrations. The observed pathological changes at lower concentrations could be attributed to the phenomenon of hormesis. Hormesis is a dose response relationship where low doses of a substance stimulate biological processes, resulting in beneficial effects, while higher doses may have toxic or inhibitory effects. In this case, the lower concentrations might trigger adaptive responses or stimulate repair mechanisms in the organism, leading to pathological changes whereas higher concentrations overwhelm these mechanisms, resulting in more pronounced toxicity without observable pathological changes. Additionally, factors such as the specific characteristic of the extract and the biological variability of the organism could also contribute to the observed results. The results of this findings do not align to earlier assertion reported by George et. al., (2015a) [32] when reporting on the acute toxic effects of Hevea brasiliensis on the gills of hatchery reared Oreochromis niloticus fingerlings and observed histological changes in the gills of the exposed organisms which were concentration dependent, George et.al., (2014a) [31] when investigating on the acute toxic effect of qua iboe light crude oil on the gills of Clarias gariepinus juveniles; Idowu et. al., (2019) [46] when studying the effect of Euphorbia hirta leaf extract on histopathology of juveniles Clarias gariepinus and George et.al., (2014b) [47] when reporting on the histopathological alterations in gills of fingerlings of Clarias gariepinus following sub-lethal acute exposure to Hevea brasiliensis.
Summary and Conclusion
The present study investigated the effects of ethanolic extract of Lunaea taraxifolia on fingerlings of Clarias gariepinus. Results indicated no mortality in the control group, but mortality rates increased with higher concentrations of the extract. Specifically, 20% mortality occurred at 22 mg/l, while 100% mortality was recorded at 23, 24 and 25 mg/l concentrations of the extract. Interestingly, pathological changes were more pronounced at lower concentrations than at higher concentrations, suggesting a potential hermetic response where low doses stimulated adverse effects while higher doses overwhelm the organism’s adaptive mechanisms. The observed mortality and pathological changes indicate potential harm to fish populations and this calls for concern. Therefore, this study strongly advocates the importance of monitoring and managing invasive species and pollutants in aquatic ecosystems to preserve their integrity and function.
Recommendations
Given the observe mortality and pathological changes in the experimental groups compared to the control group, its crucial to conduct further toxicity studies on Lunaea Taraxifolia. Specifically investigate dosage levels, exposure duration and potential mechanisms of toxicity to ascertain safe usage levels for C. gariepinus fingerlings. Additionally, consider examining histopathological changes, biochemical parameters and behavioural responses to comprehensively assess the effects of L. taraxifolia on the fish. These studies will provide essential data for regulatory decisions and safe implementation in aquaculture practices.
REFERENCES
- Adebisi AA. (2000) Population of Neglected Indigenous Leafy Vegetables among the Yoruba tribe of South West Nigeria. CERNARD Development Series 06 CERNARD, Ibadan, Nigeria:86.
- Owoeye O, Femi-Akinlosotu OM, Adejuwon SA, (2015). Launaea taraxacifolia aqueous extract attenuates cisplatin-induced neurotoxicity by decreasing oxidative stress and neuronal cell death in rats. Arch Bas Appl Med. 3:71-78.
- Schippmann U, Leaman D, Cunningham AB. (2006). A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. 1-21.
- Uniyal SK, Singh KN, Jamwal P, Lal B. (2006). Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J Ethnobiol Ethnomed.14:1–8.
- Power DM, Fuentes J, Harrison AP. (2010). A noninvasive monitoring device for anaesthetics in fish. Animal Physiology. 2: 1-23.
- Omoitoyin BO, Ogunsami AO, Adesina BT. (1999). Studies on acute toxicity of piscidal plants extract on tilapia (Sarotherodon galliceus) fingerlings. Tropic J Ani. Sci. 2:191-197.
- Oluwatoyin AS. (2011). Histopathology of Nile tilapia (Oreochromis niloticus) juveniles exposed to aqueous and ethanolic extracts of Ipomoea aquatic leaf. Int J Fish Aquac. 3:244-257.
- Wakawa AI, Audu BS, Sulaiman Y. (2018). Phytochemistry and proximate composition of root, stem bark, leaf and fruit of desert date, Balanites aegyptiaca, J Phytopharm Pharmacog Phytomed Res. 7:464-470.
- Fafioye OO, Adebisi AA, Fagade SO. (2004). Toxicity of Parkia biglobosa and Raphia vimfera extracts on Clarias gariepinus juveniles. Afr J Biotechnol. 3(11):627-630
- Fafioye OO. (2011). Lethal and sublethal effects of plant extract on some freshwater fauna. PhD thesis, University of Ibadan, Nigeria.
- Roy RK, Munshin DJ. (1989). Effects of saponin extracts in oxygen uptake and haematology of air brcathing climbina perch (Anabastes titutdines) (Bloch). J Fresh Water Biol. 1:167-172.
- Tsuchiya H. (2017). Analgestic agents of plant origin. A review of phytochemicals with anaesthetic activity. Molecules.. 22:1369-1402.
- Jonah UE, George UU. (2019). Influence of Water Quality on Zooplankton Community Structure of Etim Ekpo River, Akwa Ibom State, South-South, Nigeria. World Rural Observation. 11(3):49-57.
- Jonah UE, George UU, Avoaja DA. (2019). Impacts of Anthropogenic Perturbation on Water Quality Characteristics of Ikpe Ikot Nkon River, Southern Nigeria. New York Sci J. 12(9):70-77.
- Jonah UE, George UU, Avoaja DA. (2020). Impacts of Agrochemical on Water Quality and Macroinvertebrates Abundance and Distribution in Ikpe Ikot Nkon River, South-South, Nigeria. Researcher. 12(1):36-43.
- Salau AK, Yakubu MT, Oladiji AT. (2013). Cytotoxic activity of aqueous extracts of Anogeissus leiocarpus and Terminalia avicennioides root barks against ehrlich ascites carcinoma cells. Indian J Pharm. 45:381-385.
- George UU, Ajayi OO, George IE, Vincent UE. (2023a). Establishing a Dose-Response Toxicity for Clarias gariepinus Fingerlings Exposed to Ethanolic Extract of Latana camara. Asia J Fisheries Aquatic Res. 24(1):1-10.
- George UU, Mbong EO, Abiaobo NO, Akpan II. (2023b). In Vivo Studies on Mortality and Histopathological Indices of Phragmenthera capitata (Mistletoes) on Clarias gariepinus Fingerlings in Aquarium. Mathews J Cytol and Histol. 7(2):1-10.
- Udo PJ, Ekanem AP, Eze EE. (2006). Toxicity of crude oil to early life stages of Heterobranchus longifilis (Cruveier and Valiennces) Pisces: Bagridae). Tropical Envir Res. 1:450-459.
- Hidayat R, Wulandari P. (2021). Methods of Extraction: Maceration, percolation and Decoction. Eureka Herba Indonesia. 2(1):68-74.
- Dede EB, Kagbo HD. (2001). Aqua toxicological effects of water-soluble fraction (WSF) of diesel fuel on Oreochromis niloticus fingerlings. J Appl Sci Envir Manage. 5(1):93-96.
- APHA (American Public Health Association). (1998). Standard Methods for the examination of water and waste waters. 20th Edn. New York:314.
- Udo PJ. (2007). Techniques in Fish farming (Practice and Management). Wusen Publishers, Calabar, Nigeria:100
- Ekanem AP, Ekpo IA. (2008). Effects of commercial detergents on the juvenile of Herterobranchus longifilis (Curvier and Valiennies). Afr J Envir Pollut Health. 6(1):18-23.
- Chan EI. (1977). Oil pollution and tropical littoral communities. Biological effects of the 1975 Floride key oiul spills. Pp 187-192. In: Procedures of oil spill Conference. Api publication, Washington DC.
- Omoregie E. (2002). Acute Toxicity of Water-Soluble Fraction of Crude Oil to the Nile Tilapia (Oreochromis niloticus). Bull Environ Contam Toxicol. 68:623-629.
- Omoregie E, Ufodike BC. (2000). Effects of water-soluble fraction of crude oil on growth of the Nile Tilapia (Oreochromis niloticus) (L). Bull Environ Contam Toxicol. 64:601-605.
- Ajah PO. (2007). Fish feeding and hatchery management. Calabar, Nigeria. Jerry Commercial Productions, 178.
- George UU, Asuquo FE, Idung JU, Andem AB. (2013a). Effects of Lethal Concentration of Rubber Extract (Hevea brasiliensis) on the survival on fingerlings of Clarias gariepinus Under Laboratory Conditions. J Natural Sci Res. 3(9):56-60.
- George UU, Asuquo FE, Idung JU, Andem AB. (2013b). A Laboratory Bioassay of the Potential Effects of Rubber Extract (Hevea brasiliensis) on the survival of fingerlings of Oreochromis niloticus. J Agri Health Care. 3(11):70-74.
- George UU, Joseph A, Andy JA. (2014a). Histopathological Alterations in Gills of Fingerlings of Clarias gariepinus (Burchell, 1822) Following Sublethal Acute Exposure to Hevea brasiliensis. Inte J Sci Technol Res. 3(9):252-255.
- George UU, Etim IN, Ekanim MP, Akpan MK. (2015a). Acute Toxic Effects of Hevea brasiliensis on the Gills of Hatchery Reared Oreochromis niloticus Fingerlings. J Academia Industrial Res. 3 (11):562-566.
- George UU, Etim IN, Ekanim MP, Akpan MK. (2015 c). Toxic Effect of Crude Oil on Hatchery Reared Oreochromis niloticus Fingerlings. J Academia Ind Res. 3(11):573-576.
- George UU, Etim IN, Ekan P, Otu U. (2015b). Acute Toxicity of Ariel Detergent on the Survival of Fingerlings of Nile Tilapia (Oreochromis niloticus). Int J Current Res. 7 (4):14825-14828.
- Bossayt BA, Janssen C. (2005). Copper toxicity to different field collected Cladoceran Sp: Intra and Inter specific sensitivity. Envir Pollut. 136:145-154.
- George UU, Otoh AJ, Ajayi OO, George IE. (2023c). Studies on mortality and Histopathological Alteration on the Gills of Oreochromis niloticus Juveniles Following Exposure to Ethanolic Extract of Phramenthera capitata Under Laboratory Conditions. Asian J Fisheries Aquatic Res. 24(3):23-34.
- George UU, Otoh AJ, Ajayi OO, George IE. (2023d). Dose-Response Relationship and Histo-Morphological Alterations on Oreochromis niloticus Juveniles following Exposure to Ethanolic Extract of Latana camara. Asian J Res Zoology. 6(4):71-83.
- Ogundiran MA, Fawole OO, Aderoye SD, Ayandiran TA. (2010). Toxicological impacts of detergents effluent on juveniles of African catfish (Clarias gariepinus) (Burchell, 1822). Agri Biol J North America. 1(3):330-342.
- Calta M, Ural MS. (2004). Acute toxicity of the synthetic pyrethroid deltamethrin to young mirror carp, Cyprinus carpio. Fresen Environmental Bulletin; 13(11a):1179–1183.
- Ayuba JO, Ofojekwu PC. (2002). Acute toxicity of the root of Jimson weed (Datura innoxia) to the African catfish (Clarias gariepinus) fingerlings. J Aquatic Sci. 17:131-133.
- Adedeji BA, Adedeji AO, Adeyemo OK, Agbede SA. (2008). Acute toxicity of diaziam to the African catfish Clarias gariepinus. African J Biotechnol. 7(5): 651-654.
- Essien-Ibok MA. (2020). The Toxicity of Ethanolic Extract of Alchornea cordifolia Leaf on Clarias gariepinus Fingerlings. Asian J Envir Ecol. 12(2): 22-28.
- Lagauan AG, Galaites MC, Fajardo LJ. (2004). Evaluation of botanical piscicides on nile tilapia (Oreochromis niloticus) and mosquito fish (Gambeesia affini, Baird and Girard). Proceedings of the6 th International Symposium on tilapia in aquaculture, September 12-16, Manila, Philippines. American Tilapia Association and Philippine Bureau of Fisheries and Aquatic Resources:179-187.
- Ayotunde EO, Offem BO, Bekeh AF. (2011). Toxicity of Carica papaya Lim: Haematological and piscicidal effect on adult catfish (Clarias gariepinus). J Fisheries Aquatic Sci. 6(3):291-308.
- Ayotunde EO, Ofem BO, Okey IB, Ikpi GU, Ochang SN, Agbam NE, et al. (2010). Toxicity of pawpaw (Carica papaya) seed provide to sharp-tooth catfish (Clarias gariepinus) fingerlings and effects on haematological parameters. Int J Fisheries Aquaculture. 2:71-78.
- Idowu AA, Soetan MO, Akinde A, Popoola OC. (2019). Effect of Euphorbia hirta leaf extracts on histopathology of juvenile Clarias gariepinus. Nigerian J Ani Sci. 21 (1):96-109.
- George UU, Urom SE, Etanketuk N. (2014 b). Acute Toxic Effect of Qua Iboe Light Crude Oil on the Gills of Clarias gariepinus Juveniles. Int J Envir Pollut Res. 2(2):16-30.